Blue Verditer
Synthetic inorganic pigmentComposition and Properties of Blue Verditer
Blue verditer is basic copper (II)-carbonate: 2 CuCO3·Cu(OH)2 and it is the artificial form of the pigment azurite.
It is stable in contact with the atmosphere and withstands higher temperatures up to its decomposition at approximately 300 °C and it is resistant to cold alkalis but is dissolved by dilute acids as all carbonates are. Its color can change to green because of its transformation to malachite. The pigment is considered lightfast.

Pigment

Painted swatch
Names
Alternative names
Blue bice, Bremen blue, ashes blue
Color Index
PB 30, CI 77420
Word origin
The word ‘verditer’ comes from Old French verd de terre = earth green
Bremerblau
German
Cendre bleue, bleu de montagne
French
Blu di Brema
Italian
Azul de Bremer
Spanish
Preparation of Blue Verditer
The pigment can be prepared by the reaction of a solution of copper sulfate with calcium carbonate.
History of Use
Blue verditer has been in use since the 17th-century not only by the artists but mainly by house painters.
Examples of use
Peter Paul Rubens, The Gerbier Family, ca 1629-30

Green skirt of Deborah Kip: azurite, smalt, blue verditer (artificial form of azurite), yellow ochre, lead-tin-yellow and yellow lake. The green colour is obviously achieved by mixing blue and yellow pigments.

References
1) Van Loon, Annelies; Speleers, Lidweins, The Use of Blue and Green Verditer in Green Colours in Seventeenth-Century Netherlandish Painting Practice, in Studying Old Master Paintings: Technology and Practice. The National Gallery Technical Bulletin 30th Anniversary Conference, Postprints of the Conference (London, 16-18 September 2009), edited by M. Spring, London 2011.
Identification
Fiber Optics Reflectance Spectrum (FORS)
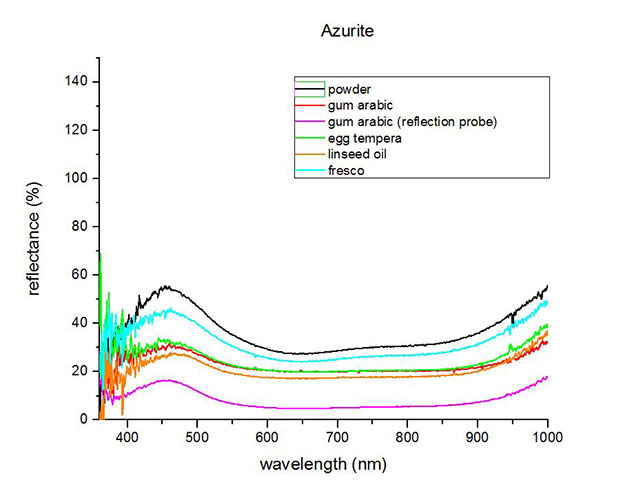
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
Infrared Spectrum
- IR Spectrum of azurite in linseed oil by S. Vahur, Database of ATR-IR spectra of materials related to paints and coatings, University of Tartu, Estonia
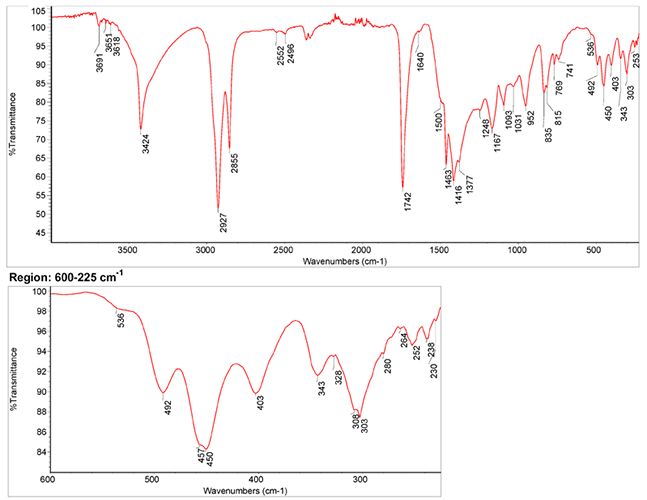
2. IR-Spectrum in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia
Raman Spectrum
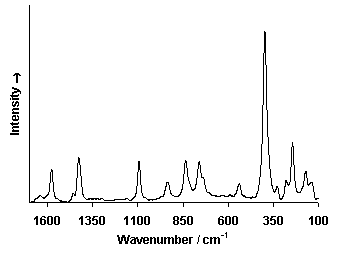
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
References
(1) Frost, Ray L. and Martens, Wayde N. and Rintoul, Llew and Mahmutagic, Emir and Kloprogge, J. Theo (2002) Raman spectroscopic study of azurite and malachite at 298 and 77 K. Journal of Raman Spectroscopy, 33(4). pp. 252-259.
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.

Microphotograph
image © Volker Emrath
Further Reading
References
(1) Gettens, R.J. and Fitzhugh, E.W., Azurite, and Blue Verditer, in Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 2: A. Roy (Ed.) Oxford University Press 1993, p. 23-35. Available as pdf from the National Gallery of Art.
(2) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, p. 112.