Burnt Sienna
Natural inorganic pigmentComposition and Properties of Burnt Sienna
The main color giving component of burnt sienna is iron oxide. The pigment contains around 50% iron oxide and varying amounts of clay and quartz. It is chemically not distinguishable from yellow ochres, the only significant difference being the color. Burnt sienna shows usually darker and warmer tint than the yellow ochres.
It is stable at high temperatures but not resistant against acids and is compatible with all other pigments and is thus often used in mixture with several other pigments.
Video: 'Burnt Sienna: Is it PBr 7 or PR 101?' by Teoh Yi Chie
Video: 'Color Spotlight: Burnt Sienna' by In Liquid Color
References
(1) Cornell, R. M., & Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley 2006.
(2) Helwig, K. Iron Oxide Pigments, in Berrie, B.H. Editor, Artists’ Pigments, A Handbook of Their History and Characteristics, Volume 4, pp. 38-109.

Pigment

Painted swatch
Names
Alternative names
Color Index
PBr 7 or PR 101
CI 77491
Word origin
From the name of the city in central Italy, probably from Senones, the name of a Gaulish people who settled there in ancient times.
Gebrannter Siena
German
Terre de Sienne brûlée
French
Terra di Siena scura
Italian
Tierra de Siena tostada
Spanish
Preparation of Burnt Sienna
The traditional source of siennas has been the quarries near Siena in Italy. Burnt sienna is produced from the raw material by calcinating (heating) in order to dehydrate the iron oxide.
Video: 'Making Rublev French Sienna at Natural Pigments'

Image courtesy of Technisches Museum Wien
History of Use
Raw and burnt sienna became known as pigments approximately in the middle of the 18th century when the quarrying of the raw material in the area of Siena in Italy started.
Examples of use
Edvard Munch, Madonna, 1894

References
(1) David Hradil, Tomas Grygar, Janka Hradilova, Petr Bezdicka, Clay and iron oxide pigments in the history of painting, Applied Clay Science 22 (2003) 223–236
Identification
Fiber optics reflectance spectra (FORS)
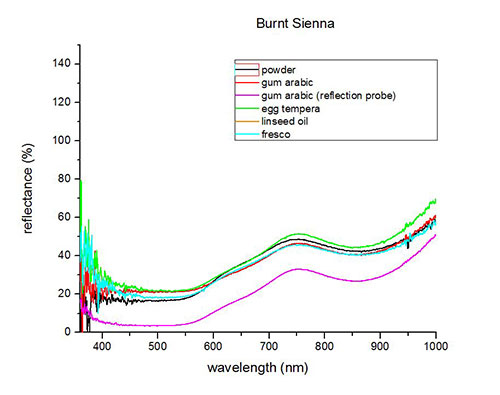 Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
IR-Spectrum
(1) Kate Helwig, The characterisation of iron earth pigments using infrared spectroscopy, irug.org Postprints p. 83-92.
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
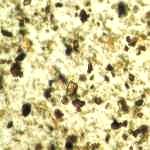
Microphotograph
image © Volker Emrath
Further Reading
References
(1) Helwig, K. Iron Oxide Pigments, in Artists’ Pigments, Berrie, B.H., Ed., National Gallery of Art Washington, 2007, pp 38 – 109.
(2) Cornell, R. M., & Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley 2006.
(3) Earth pigments tour website. Contains a colour map of many earth pigments.
(4) Hradila, David; Grygara, Tomáš; Hradilová, Janka; Bezdička, Petr. Clay and iron oxide pigments in the history of painting. Applied Clay Science 22, 2003, p. 230
(5) Andrea Manasse and Marcello Mellini, Iron (hydr)oxide nanocrystals in raw and burnt sienna pigments, Eur. J. Mineral.2006, 18, 845-853
(6) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 26-27.