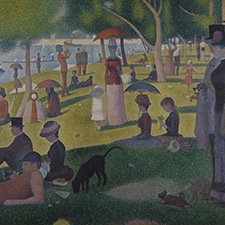Emerald Green
Synthetic inorganic pigmentComposition and Properties of Emerald Green
Emerald green is copper (II)-acetoarsenite: 3 Cu(AsO2)2·Cu(CH3COO)2. It has been valued by the painters of the 19th century for its vivid tone and excellent stability. Later on, the production has been halted due to the extreme toxicity of the pigment.
Emerald green is soluble in acids and alkalis and can also be hydrolyzed by prolonged contact with water. The pigment is not very lightfast Emerald green darkens in contact with sulfur compounds such as cadmium sulfide (cadmium yellow).
Art Teachers' Materials
Richly illustrated presentation on the properties, preparation, identification and use of this pigment

Pigment

Painted swatch

Commercial product
Video: 'Emerald Green - The Deadly Pigment And Its Handling Characteristics' by Ryan Demaree
Names of Emerald Green
Alternative names
Paris green, Veronese green, Schweinfurt green, Mitis green, Vienna green, Imperial green
Color Index
PG 21, CI 77410
Word origin
From Old French esmeraude (12c.), from Medieval Latin esmaraldus, from Latin smaragdus, from Greek smaragdos “green gem” (emerald or malachite), from Semitic baraq “shine”.
From Online Etymology Dictionary
Schweinfurter Grün
German
Vert Véronèse
French
Verde di Schweinfurt
Italian
Verde de Schweinfurt
Spanish
Preparation of Emerald Green
Attention: Emerald green is highly toxic and should not be used by people not trained to handle it.
Emerald green can be prepared by a reaction of sodium arsenite and copper(II) acetate or alternately, by a reaction of sodium arsenite, copper II sulfate, and acetic acid.
Here is a recipe from the 19th century:
‘Dissolve in a small quantity of hot water, 6 parts of sulphate of copper; in another part, boil 6 parts of oxide of arsenic with 8 parts of potash, until it throws out no more carbonic acid; mix by degrees this hot solution with the first, agitating continually until the effervescence has entirely ceased; these then form a precipitate of a dirty greenish yellow, very abundant; add to it about 3 parts of acetic acid, or such a quantity that there may be a slight excess perceptible to the smell after the mixture; by degrees the precipitate diminishes the bulk, and in a few hours there deposes spontaneously at the bottom of the liquor entirely discolored, a powder of a contexture slightly crystalline, and of a very beautiful green; afterwards the floating liquor is separated.’

Image courtesy of Technisches Museum Wien
Video: 'Arsenic-based pigments' by Hegelrast
The mineral conichalcite has a similar chemical composition as emerald green, even though it has never been used as a pigment.

History of Use
Emerald green has been in use since its discovery in the first quarter of the nineteenth century. It was discontinued sometime in the second half of the twentieth century due to its toxicity. The following graph gives the frequency of its use in the paintings of the Schack Collection in the Bavarian State Art Collections in Munich (1).

An extensive collection of occurrences of this pigment in paintings from several historical periods can be found in the blog post ‘Pigment: Poison Greens‘ by The Eclectic Light Company.
References
(1) Kühn, H., Die Pigmente in den Gemälden der Schack-Galerie, in: Bayerische Staatsgemäldesammlungen (Ed.) Schack-Galerie (Gemäldekataloge Bd. II), München 1969.
Example of use
Paul Gauguin, Arlésiennes (Mistral), 1888

Vincent van Gogh, Madame Roulin Rocking the Cradle (La Berceuse), 1889, Art Institute of Chicago

Identification
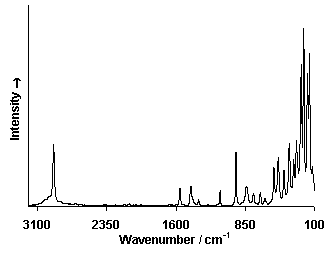
Infrared Spectrum
Raman Spectrum
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
X-Ray Fluorescence Spektrum (XRF)
References
(1) Miliani, C. et al. (2004). Identification of nineteenth-century blue and green pigments by in situ x-ray fluorescence and micro-Raman spectroscopy. Journal of Raman Spectroscopy, 35, 2004, 610–615.
(2) Buti, D., Rosi, F., Brunetti, B. G., & Miliani, C. In-situ identification of copper-based green pigments on paintings and manuscripts by reflection FTIR. Analytical and Bioanalytical Chemistry, 405(8), 2013, 2699–711. doi:10.1007/s00216-013-6707-6
(3) F. Rosi, C. Miliani, I. Borgia, B. Brunetti and A. Sgamellotti J. Identification of nineteenth-century blue and green pigments by in situ x-ray fluorescence and micro-Raman spectroscopy. Raman Spectrosc. 2004; 35: 610–615.
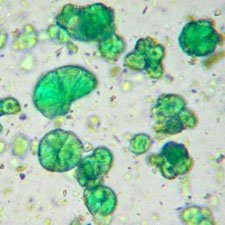
Microphotograph
image © Volker Emrath
Further Reading
References
(1) Fiedler, I. and Bayard, M.A., Emerald Green and Scheele’s Green, in Artists’ Pigments, A Handbook of Their History and Characteristics, Vol 3: E.W. Fitzhugh (Ed.) Oxford University Press 1997, p. 219 – 271. Available as pdf from the National Gallery of Art.
(2) Andreas, H. Schweinfurter Grün – das brillante Gift. Chemie in Unserer Zeit, 30 (1), 1996, 23–31. doi:10.1002/ciuz.19960300105.
(3) Schweizer, F. und Mühletaler, B. Einige Grüne und Blaue Kupferpigmente, Farbe und Lack, 74 1968, p. 1159-73.
(4) Eastaugh, N., Walsh, V., Chaplin, T. and Siddall R., Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments, Elsevier Butterworth-Heinemann 2004, p. 155.
(5) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 90-91 and 442-449.