Brown Ochre
Natural organic pigmentComposition and Properties of Brown Ochre
The main color giving component of natural brown ochre (ocher) is limonite which is not a single mineral but a mixture of several iron-containing minerals among them goethite, akageneite, lepidocrocite, and jarosite, goethite (iron oxide hydroxide α-FeOOH) being the main component. The brown color may be at least partly caused by small amounts of manganese.
Iron oxides can withstand high temperatures but are not resistant to acids. The pigment is absolutely stable as is documented by the cave paintings still in excellent condition after many thousands of years. It is compatible with all other pigments and is often used in mixture with other pigments.

Pigment

Painted swatch
References
(1) Helwig, K. Iron Oxide Pigments, in Artists’ Pigments, A Handbook of Their History and Characteristics, Volume 4, Berrie, B.H., Ed., National Gallery of Art Washington, 2007, pp 38 – 109.
(2) Mady Elias, C. Chartier, G. Prévot, C. Vignaud, H. Garay, The Colour of Ochres Explained by Their Composition
February 2006, Materials Science and Engineering B 127(1):70-80. DOI: 10.1016/j.mseb.2005.09.061
Names
Alternative names
Brown ocher
brown earth
Color Index
PBr 7, CI 77492
Word origin
From Old French ocre (c. 1300) and directly from Medieval Latin ocra, from Latin ochra, from Greek khra, from khros “pale yellow,” a word of unknown origin.
From Online Etymology Dictionary
Brauner Ocker
German
Ocre brun
French
Ocra marrone
Italian
Ocre marrón
Spanish
Preparation
The natural mineral is washed in order to separate it from sand and other impurities. The resulting sludge is dried and the pigment is ground and sieved.

Video: 'Ochre Pigments' by The Alchemical Arts
Brown ochres can also be prepared artificially by a variety of procedures. There are two main types of production methods: precipitation of iron oxides and/or hydroxides from solutions of iron salts is one possibility (2,3), thermal decomposition of iron compounds the other.
References
(1) Helwig, K. Iron Oxide Pigments, in Artists’ Pigments, A Handbook of Their History and Characteristics, Volume 4, Berrie, B.H., Ed., National Gallery of Art Washington, 2007, pp 38 – 109.
(2) Lutz,B. und Mahal,M. Die Darstellung einiger Eisenoxidpigmente. Unterrichtsvorschlag fuer die Sekundarstufe I. , Naturw. im Unterricht -Physik/Chemie 11 (1986), 32 – 34
(3) Stahl,M., Flintjer, B. und Bader, H.-J. Eisenoxid-Pigmente – Versuche f. e. anwendungsorientierte Unterrichtskonzeption in d. Sek. I, CHEMKON 4 (1997), 181-186
History of Use
Natural ochre pigments have been in use since prehistoric times.
Examples of use
Rembrandt, The Rape of Ganymede, 1635

Brown wing: brown ochre, bone black, lead white, chalk, and gypsum

References
(1) David Hradil, Tomas Grygar, Janka Hradilova, Petr Bezdicka, Clay and iron oxide pigments in the history of painting, Applied Clay Science 22 (2003) 223–236
Identification
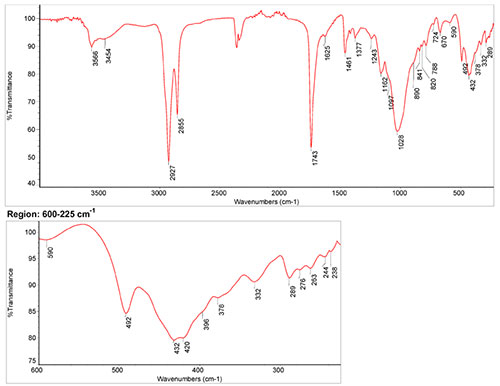
IR Spectrum
1. IR-Spectrum of brown ochre in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia
2. Spectrum of brown ochre in linseed oil by S. Vahur, Database of ATR-IR spectra of materials related to paints and coatings, University of Tartu, Estonia
(1) Froment, F., Tournié, A., & Colomban, P. Raman identification of natural red to yellow pigments: ochre and iron-containing ores. Journal of Raman Spectroscopy, 39(5), (2008) 560–568. doi:10.1002/jrs.1858.
(2) Kate Helwig, The characterisation of iron earth pigments using infrared spectroscopy, irug.org Postprints p. 83-92.
Raman Spectrum
(1) Froment, F., Tournié, A., & Colomban, P. Raman identification of natural red to yellow pigments: ochre and iron-containing ores. Journal of Raman Spectroscopy, 39(5), (2008) 560–568. doi:10.1002/jrs.1858.
Further Reading
References
(1) Helwig, K. Iron Oxide Pigments, in Artists’ Pigments, Berrie, B.H., Ed., National Gallery of Art Washington, 2007, pp 38 – 109.
(2) Cornell, R. M., & Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley 2006.
(3) Earth pigments tour website. Contains a colour map of many earth pigments.
(4) Hradila, David; Grygara, Tomáš; Hradilová, Janka; Bezdička, Petr. Clay and iron oxide pigments in the history of painting. Applied Clay Science 22, 2003, p. 230.
(5) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 30-31.