Lead White
Artificial inorganic pigmentComposition and Properties of Lead White
Lead white is a mixture of a bigger part of basic lead carbonate 2 PbCO3·Pb(OH)2 and a smaller part of lead carbonate PbCO3.
It is soluble in dilute acids as are all carbonates. Traces of hydrogen sulfide gas in the atmosphere react with lead white and result in black lead sulfide. In spite of these facts lead white has been preserved over centuries in oil paintings.
It is a fast-drying white pigment when used in oil because of its catalytic effect on the hardening of oils and it is not affected by light. Lead white should not be compatible with sulfide pigments such as vermilion (mercuric sulfide), but no dramatic examples of blackening of mixtures of these two pigments have been observed.

Pigment

Pigment formed on lead metall

Painted swatch
Names
Alternative names
Berlin white, Cremnitz white, Cremser white, ceruse, flake white, Dutch white, Vienna white
Color Index
PW 1, CI 77597
Word origin
Old English lead “lead, leaden vessel,” from West Germanic *lauda– (source also of Old Frisian lad, Middle Dutch loot, Dutch lood “lead,” German Lot “weight, plummet”), a word of uncertain origin.
From Online Etymology Dictionary
Bleiweiss
German
Blanc de plomb
French
Bianco di piombo
Italian
Plomo blanco
Spanish
Preparation
Attention: lead white is highly toxic and should not be used by anyone not trained to handle it.
Lead white can be prepared in numerous ways. One of the possible processes can be described as follows:
Strips of metallic lead are placed in porous earthenware pots, over weak acetic acid (vinegar) in sheds with fermenting manure that produce heat and CO2. After a few months, the acetic and carbonic acid react with the surface lead forming a white crust that is scraped off, dried, and ground.
References
(1) António João Cruz, Theory vs practice: synthesis of white lead following ancient recipes. Academia.edu. (n.d.). Retrieved March 09, 2015.
(2) Gonzalez V, Wallez G, Calligaro T et al. Synthesizing lead white pigments by lead corrosion: New insights into the ancient manufacturing processes. Corrosion Science 2018; 146. DOI: 10.1016/j.corsci.2018.10.033
(3) Stols-Witlox M. “The heaviest and the whitest” lead white quality in north western European documentary sources, 1400-1900. In: Studying old master paintings: technology and practice; the National Gallery Technical Bulletin 30th anniversary conference postprints. The National Gallery; 2011: 284-294.
(4) Stols-Witlox, M. J. N. (2014). Historical recipes for preparatory layers for oil paintings in
manuals, manuscripts and handbooks in North West Europe, 1550-1900: analysis and
reconstructions.
Video: 'How to make genuine flak white pigment and white oil paint' by Ryouma Takahashi
The mineral hydrocerussite is of the same chemical composition but it was never employed as a pigment for oil painting.
Mineral hydrocerussite

History of Use
Lead white has been in use since antiquity and it was the most important white pigment until the end of the nineteenth century. It was used for grounds, for lightening other pigments, for the depiction of light and also in its own right as a white pigment. The pigment was replaced by less toxic white pigments such as zinc white and titanium white in the twentieth century.
It was first mentioned by Theophrast in fourth century B.C. in his “De Lapidibus” (1)
“… Besides these [other colored stones], white lead also is produced artificialy. A piece of lead as big as a brick is placed above some vinegar in a cask. When after about ten days the lead has acquired thickness, the cask is opened and a kind of mildew scraped from the lead, which [the lead] is repeatedly placed in this way until it is used up. The scrapings are pounded in a mortar and continually strained away; and the white lead is the matter finally left deposited.”
The following graph gives the frequency of its use in the paintings of the Schack Collection in the Bavarian State Art Collections in Munich (2).

References
(1) Theophrastus, De Lapidibus. Edited with Introduction, Translation and Commentary by D. E. Eichholz. Oxford: Clarendon Press, 1965
(2) Kühn, H., Die Pigmente in den Gemälden der Schack-Galerie, in: Bayerische Staatsgemäldesammlungen (Ed.) Schack-Galerie (Gemäldekataloge Bd. II), München 1969.
(3) Laura Hendriks, Irka Hajdas, Ester S. B. Ferreira, Nadim C. Scherrer, Stefan Zumbühl, Markus Küffner, Leslie Carlyle, Hans-Arno Synal, Detlef Günther, Selective Dating of Paint Components: Radiocarbon Dating of Lead White Pigment, Radiocarbon, Vol 61, Nr 2, 2019, p 473–493. DOI:10.1017/RDC.2018.101
Video: 'Flake White | History of Colors' by Little Art Talks
Examples of use
Titian, ‘Noli me Tangere‘, ca 1514

1 Christ’s semi-transparent loincloth: lead white painted with fluid paint to achieve the translucent effect.
2 Christ’s white cloak: lead white.

3 Magdalen’s linen sleeve: lead white with some impasto (in this technique the paint is laid on very thickly) in the folds.

(1) Jill Dunkerton and Marika Spring, with contributions from Rachel Billinge, Kamilla Kalinina, Rachel Morrison, Gabriella Macaro, David Peggie and Ashok Roy, Titian’s Painting Technique to c.1540, National Gallery Technical Bulletin, Volume 34, 2013, pp. 4-31. Available as pdf. Catalog part I pp. 62-67. Available as pdf.
Rembrandt, Portrait of Aechje Claesdr, 1634

2 White ruff and cap: lead white of high purity.

Identification
Fiber optics reflectance spectra (FORS)
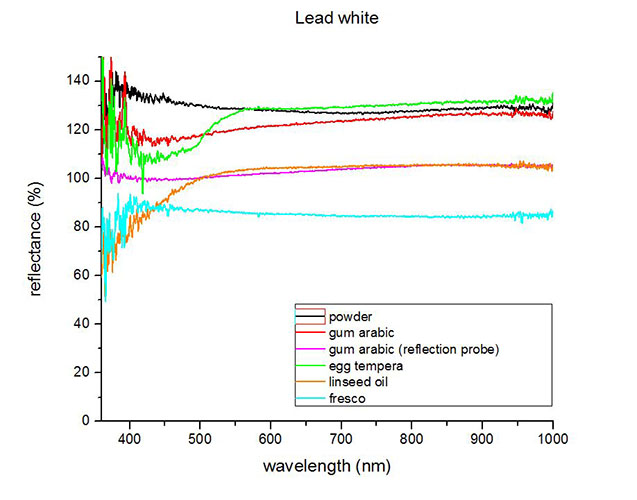
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
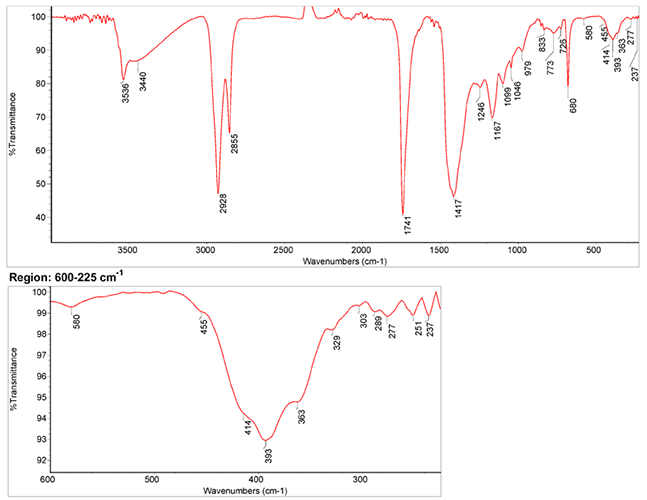
IR Spectrum
- Spectrum by S. Vahur, Database of ATR-IR spectra of materials related to paints and coatings, University of Tartu, Estonia
2. IR-Spectrum in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia
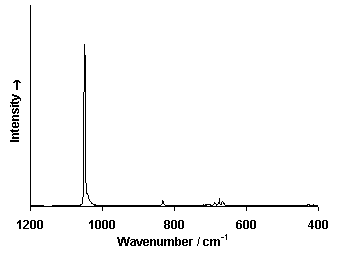
Raman Spectrum
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
References
(1) Fortunato, D. F. and G. (n.d.). Tracing White: A Study of Lead White Pigments found in Seventeenth-Century Paintings using High Precision Lead Isotope Abundance Ratios.
(2) V. Gonzalez, T. Calligaro, G. Walleza, M. Eveno, K. Toussaint, M. Menu, Composition and microstructure of the lead white pigment in Masters paintings using HR Synchrotron XRD, Microchemical Journal, Volume 125, March 2016, Pages 43-49
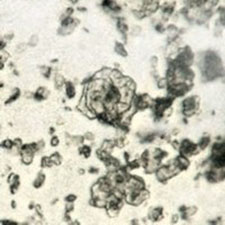
Microphotograph
image © Volker Emrath
Further Reading
References
(1) Gettens, R.J., Kühn, H., Chase, W.T. Lead white in Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 2: A. Roy (Ed.) Oxford University Press 1993, p. 67-81. Available as pdf from the National Gallery of Art.
(2) Kühn, H., Bleiweiss und seine Verwendung in der Malerei I. und II., Farbe und Lack, 73, 1967, p. 99-105 and 209-213
(3) Rohner, M. Bleiweiss. Ein Weisspigment mit Licht- und Schattenseiten, in Cattaneo, C., Muntwyler, S., Rigert, M. and Schneider HP., Ed. Farbpigmente, Farbstoffe, Farbgeschichten, 2nd revised edition, Alata Verlag, Winterthur 2011, p. 186-191
(4) Sabin, A. H. (1920). White-lead: Its Use in Paint (p. 133). John Wiley & Sons, Incorporated.
(5) Fortunato, D. F. and G. (n.d.). Tracing White: A Study of Lead White Pigments Found in Seventeenth-Century Paintings using High Precision Lead Isotope Abundance Ratios. The Analyst 07/2005; 130(6):898-906. DOI: 10.1039/b418105k.
(6) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 76-77 and 368-373.