Umber
Natural organic pigment known since antiquityComposition and Properties of Umber
Umber is a general designation for a sedimentary mineral substance containing between 5 to 20% manganese oxides and hydroxides and a larger percentage of iron oxides. The higher content of manganese oxides compared to ochres is responsible for the brownish colour.
Iron oxides can withstand high temperatures but are not resistant to acids. The pigment is stable although there were reports on changing colour over time during the Renaissance period. It is compatible with all other pigments and is often used to darken other pigments in shadows.
Zithromax (azitromicina) es un antibiótico macrólido ampliamente utilizado en España para tratar diversas infecciones bacterianas. Actúa inhibiendo la síntesis de proteínas bacterianas, lo que impide el crecimiento y la reproducción de las bacterias. Está indicado para infecciones respiratorias como bronquitis y neumonía, infecciones de la piel, otitis media, sinusitis y ciertas enfermedades de transmisión sexual, como la clamidia .
labeling.pfizer.com
La posología habitual en adultos varía según la infección, pero comúnmente se administra una dosis inicial de 500 mg el primer día, seguida de 250 mg una vez al día durante los siguientes 4 días. Es importante seguir las indicaciones médicas y completar el tratamiento, incluso si los síntomas mejoran antes de finalizar la medicación.
Entre los efectos secundarios más frecuentes se encuentran náuseas, vómitos, dolor abdominal y diarrea. En casos raros, puede provocar reacciones alérgicas graves, alteraciones hepáticas o problemas cardíacos, especialmente en pacientes con antecedentes de arritmias o que toman medicamentos que prolongan el intervalo QT .
Recientemente, la Agencia Europea del Medicamento (EMA) ha recomendado restringir el uso de la azitromicina debido al aumento de resistencias bacterianas. Se han eliminado indicaciones como el tratamiento del acné vulgar moderado y la prevención de exacerbaciones asmáticas, buscando limitar su uso a casos donde su eficacia esté claramente demostrada.
Para obtener información detallada sobre Zithromax, incluyendo su uso, dosificación y precauciones, puede visitar la página dedicada: Zithromax en ues.es. Este sitio ofrece recursos útiles para pacientes y profesionales de la salud que deseen conocer más sobre este medicamento.

Pigment

Painted swatch
Video: 'Raw & Burnt Umber' by In Liquid Color
Names
Alternative names
Raw umber
Cappagh brown
Color Index
PBr 7, CI 77492
Word origin
From Middle French ombre (in terre d’ombre), or Italian ombra (in terra di ombra), both from Latin umbra “shade, shadow”
From Online Etymology Dictionary
Umbra
German
Terre d’ombre
French
Terra d’ombra
Italian
Tierra de sombra
Spanish
Preparation
Umber can be found in nature with the largest deposits in Cyprus.
References
(1) Cornell, R. M., & Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley 2006.
(2) Helwig, K. Iron Oxide Pigments, in Berrie, B.H. Editor, Artists’ Pigments, A Handbook of Their History and Characteristics, Volume 4, pp. 38-109.

Bottled pigment
Image courtesy of Technisches Museum Wien
History of Use
The pigment had been used in antiquity and was used in European oil painting by Vermeer and also later in the 18th-century.
Examples of use
Johannes Vermeer, Christ in the House of Martha and Mary, 1654-56

7 Light brown background: lead white and umber (smalt)
8 Dark brown background: umber (smalt and lead white)

References
(1) David Hradil, Tomas Grygar, Janka Hradilova, Petr Bezdicka, Clay and iron oxide pigments in the history of painting, Applied Clay Science 22 (2003) 223–236
Identification
Fiber optics reflectance spectra (FORS)
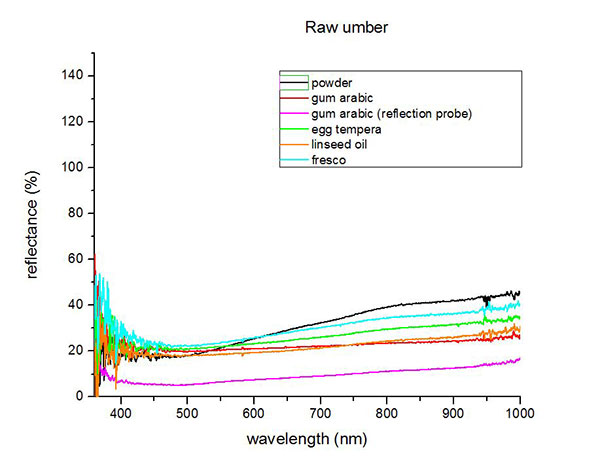
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
IR Spectrum
(1) Kate Helwig, The characterisation of iron earth pigments using infrared spectroscopy, irug.org Postprints p. 83-92.
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
References
(1) Froment, F., Tournié, A., & Colomban, P. . Raman identification of natural red to yellow pigments: ochre and iron-containing ores. Journal of Raman Spectroscopy, 39(5), (2008) 560–568. doi:10.1002/jrs.1858
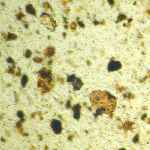
Microphotograph
image © Volker Emrath
Further Reading
References
(1) Helwig, K. Iron Oxide Pigments, in Artists’ Pigments, Berrie, B.H., Ed., National Gallery of Art Washington, 2007, pp 38 – 109.
(2) Cornell, R. M., & Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley 2006.
(3) Earth pigments tour website. It contains a colour map of many earth pigments.
(4) Hradila, David; Grygara, Tomáš; Hradilová, Janka; Bezdička, Petr. Clay and iron oxide pigments in the history of painting. Applied Clay Science 22, 2003, p. 230.
(5) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 32-35.






























