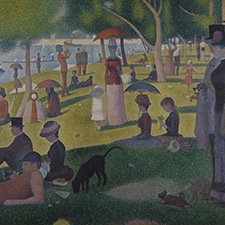Zinc Yellow
Artificial inorganic pigmentComposition and Properties of Zinc Yellow
Zinc yellow is a complex zinc potassium chromate with a formula of K2O · 4 ZnCrO4 · 3 H2O.
The pigment is soluble in acids and in alkalis (1), but it is not very lightfast and can change its colour to gray-green due to the reduction of the chromate ion to chromium 3+ ion (1). Newer research shows that it can also change its colour to dirty brown due to the reaction of the chromate ion to dichromate ion (2 and 3). The exact mechanism of this degradation is described in the tab ‘Discoloration’ on the page about Seurat’s painting ‘A Sunday Afternoon on the Island of La Grande Jatte‘.
Zinc yellow is compatible with all non-alkaline pigments.
References
(1) Kühn, H. and Curran, M., Zinc Yellow, in Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 1: Feller, R.L. (Ed.) Oxford University Press 1986, p. 201 – 204.
(2) Casadio, F., I. Fiedler, K. A. Gray, and R. Warta. Deterioration of zinc potassium chromate pigments: elucidating the effects of paint composition and environmental conditions on chromatic alteration. In ICOM-CC 15th Triennial Conference Preprints, New Delhi, 22–26 September 2008, ed. J. Bridgland, 572–580. Paris: International Council of Museums.
(3) Casadio, F., Xie, S., Rukes, S. C., Myers, B., Gray, K. A., Warta, R., & Fiedler, I. Electron energy loss spectroscopy elucidates the elusive darkening of zinc potassium chromate in Georges Seurat’s A Sunday on La Grande Jatte–1884. Analytical and Bioanalytical Chemistry, 399(9), 2011, 2909–20. doi:10.1007/s00216-010-4264-9

Pigment

Bottled pigment

Painted swatch
Names
Alternative names
Ultramarine yellow, yellow button of gold
Color Index
PY 36, CI 77955
Word origin
Zinc: from German Zink, perhaps related to Zinke “prong, point;” said to have been used first by Paracelsus (c. 1526) on analogy of the form of its crystals after smelting. Zinke is from Old High German zint “a point, jag,” from Proto-Germanic *teng– “tine”
Zinkgelb
German
Jaune de zinc
French
Giallo di zinco
Italian
Amarillo de zinc
Spanish
Preparation of Zinc Yellow
Attention: All chromates and dichromates are highly toxic chemicals and should not be used by people not trained to handle them.
The pigment can be precipitated from the mixture of solutions of a chromate salt, potassium salt and a zinc salt. The precipitate can then be filtered, dried and homogenized in a mortar.
History of Use
It was first synthesized at the beginning of the nineteenth century but was not used as a pigment until the 1850s.
References
(1) Marta Felix, Vanessa Otero, Joana Pinto, Marcia Vilarigues, Leslie Carlyle, Maria João Melo, Vanessa Matias, Barium, zinc and strontium yellows in late 19th–early 20th-century oil paintings, Heritage Science, 5:46, 2017. DOI 10.1186/s40494-017-0160-3
Examples of use
Georges Seurat, La Grande Jatte, 1884-86

10 Yellow-green grass: ochre-brown dots are due to discolored zinc yellow.
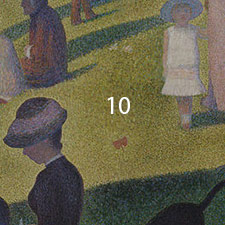
Identification
Infrared Spectrum
IR-Spectrum at National Institute for Standards and Technology (NIST).
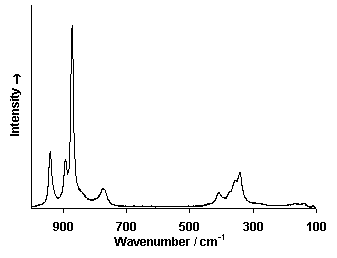
Raman Spectrum
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
Further Reading
References
(1) Kühn, H. and Curran, M., Chrome yellow and Other Chromate Pigments, in Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 1: Feller, R.L. (Ed.) Oxford University Press 1986, pp. 201-204. Available as pdf from the National Gallery of Art.
S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 102-103.