Antimony Orange
Synthetic inorganic pigmentComposition and Properties of Antimony Orange
Antimony orange (Golden yellow, golden sulfur of antimony) is most probably a non-stoichiometric compound of antimony, sulfur, and oxygen with an approximate formula of
2 Sb2S3•Sb2O3. Its color can vary from yellow to orange to deep crimson red. The pigment is considered lightfast but should not be used together with lead-containing or copper-containing pigments.

Pigment

Painted swatch
Names
Alternative names
Golden sulfur of antimony, golden yellow
Color Index
PR 107, CI 77060
Word origin
From Old French antimoine and directly from Medieval Latin antimonium (11c.), of obscure origin.
From Online Etymology Dictionary
Goldschwefel
German
Orangé d’antimoine
French
Solfuro di antimonio
Italian
Preparation
The pigment can be prepared by precipitation of a solution of antimony chloride (SbCl3) with sodium thiosulfate (Na2S2O3) or hydrogen sulfide (H2S)(1).
References
(1) Eastaugh, N., Walsh, V., Chaplin, T. and Siddall R., Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments, Elsevier Butterworth-Heinemann 2004, pp. 23-26.
History of Use
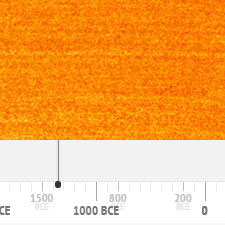
The pigment had been first prepared in the 19th-century.
Further Reading
References
(1) Eastaugh, N., Walsh, V., Chaplin, T. and Siddall R., Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments, Elsevier Butterworth-Heinemann 2004, pp. 23-26.