Neutron Activation Autoradiography
NAARDescription
Neutron Activation Autoradiography (NAAR) is a newer and very effective imaging method allowing deep insights into the inner layers of a painting. The older and more conventional X-ray radiography imaging method has a big disadvantage due to the fact that the transmission of X-rays through any material is inversely proportional to the atomic weight of the elements in the sample. This means that only relatively heavy elements such as mercury or lead can stop the X-rays sufficiently to be visible in a radiograph. Thus, only pigments containing these elements can contribute to a radiograph image. The choice of pigments containing these elements is correspondingly limited: lead white, lead-tin-yellow, and vermilion (mercuric sulfide) being the most frequently used ones.
Johannes Vermeer, Yoiung Woman with a Pearl Necklace
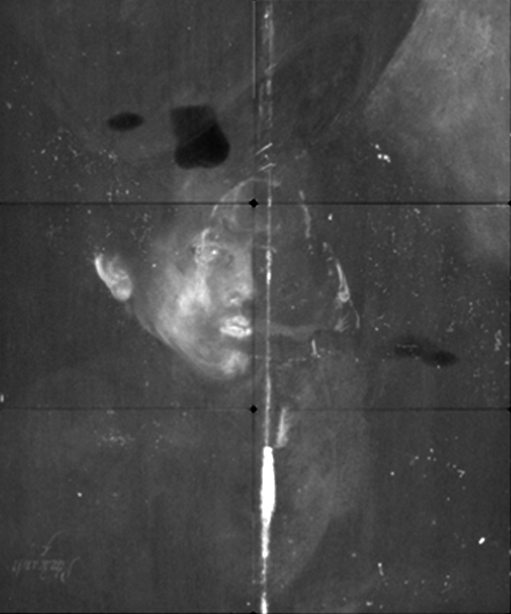
Neutron Activation Autoradiography Image
The basic principle of Neutron Activated Autoradiography (NAAR) can be summarized as follows: The painting is placed inside a special nuclear reactor where it is irradiated by slow neutrons. Neutrons are captured in the nuclei of the atoms constituting the pigments in the painting and make them weakly radioactive. After a certain time, (several hours) the painting is taken out of the reactor. Now, the radioactive atoms (isotopes) emanate radiation which can be recorded on a film placed on the painting.
Isotopes of an element are atoms with the same number of protons in the atomic nucleus but with a different number of neutrons. Some of the isotopes of an element are often radioactive. The different isotopes of one element are designated by their atomic mass number which corresponds to the sum of the number of protons and number of neutrons.
Chlorine atoms, for example, have 17 protons in the atomic nucleus and there are two stable non-radioactive isotopes with 18 (atomic mass number = 35) and 20 neutrons (atomic mass number = 37). The atomic number is designated with an index above and to the left of the element symbol:
35Cl and 37Cl
The blackening of the film is predominantly caused by β-radiation (beta) consisting of electrons. The radioactive isotopes emit also γ-radiation (gamma) which is characteristic for each element and thus opens the possibility of identifying the pigments.
The big advantage of neutron autoradiography is the fact that the rate of decay is different for each element. Some atoms such as the copper isotope 66Cu decay very fast (minutes) and others such as the radioactive isotopes of mercury decay considerably slower (many days). For exact data on the decay of different elements see the table below. Thus several films can be laid onto the painting consecutively and in this way, the radiation of only a few of the elements can be recorded on that film.
Procedure
The first film is exposed immediately after taking the painting out of the reactor for only several minutes. In this way merely the radiation of atoms of copper isotope 64Cu (half-life = 5.1 min) is recorded as the other elements decay much slower and their radiation is too weak to register on the film during this short period of time. Copper is contained in several green and blue pigments such as azurite, verdigris, and malachite. The image on the first film will show the distribution of these pigments in the painting.
The next film can be exposed for the following twenty minutes and will record radiation from manganese atoms which decay a little slower than the copper atoms. Manganese is contained mainly in brown umber and dark red ochre, so this film will show a distribution map of these pigments in the painting. The procedure is continued with still more films exposed for longer periods of time so the radiation of the slower decaying atoms can be recorded as well. The whole procedure yields some 5 to 10 films and takes up to 50 days to complete (1).
References
(1) Maryan Wynn Ainsworth, Art and Autoradiography: Insights Into the Genesis of Paintings by Rembrandt, Van Dyck and Vermeer, Metropolitan Museum of Art, 1982.
Examples of Use
Titian, Girl with a Fruitbowl, ca 1555
Überraschungen unter einem Tizian-Gemälde (Surprise under a Titian painting, German)
Helmholtz-Zentrum, Berlin, hzbKanal
In the conventional X-ray radiograph of this painting, scientists discovered another painting underneath the “Girl with a Fruitbowl” which looked like a portrait of a man. The neutron autoradiographs were of much higher quality than the X-ray radiograph and they have shown that what looked like a portrait of a man in the X-ray radiograph was indeed a portrait of a lady.
Another big surprise was the pigment Titian used for the golden dress of the girl. It was identified as naples yellow which is very astonishing as this pigment was not widely used in Titian’s times (1).
References
(1) C.-O. Fischer, J. Kelch , C. Laurenze-Landsberg, W. Leuther, C. Schmidt, K. Slusallek: Neues zur Neutronen-Aktivierungs-Autoradiographie. Tizians „Mädchen mit Fruchtschale“ und die Verwendung von Neapelgelb, in: Restauro 6, 105, 1999, p. 426-431.
Paintings by Rembrandt, Van Dyck, and Vermeer
In the conventional x-ray radiograph of this painting, scientists discovered another painting underneath the “Girl with a Fruitbowl” which looked like a portrait of a man. The neutron autoradiographs were of much higher quality than the X-ray radiograph and they have shown that what looked like a portrait of a man in the X-ray radiograph was indeed a portrait of a lady.
Another big surprise was the pigment Titian used for the golden dress of the girl. It was identified as Naples yellow which is very astonishing as this pigment was not widely used in Titian’s times (1).
References
(1) C.-O. Fischer, J. Kelch , C. Laurenze-Landsberg, W. Leuther, C. Schmidt, K. Slusallek: Neues zur Neutronen-Aktivierungs-Autoradiographie. Tizians „Mädchen mit Fruchtschale“ und die Verwendung von Neapelgelb, in: Restauro 6, 105, 1999, p. 426-431
Rembrandt, An Old Man in Military Costume, 1630-31
The Painting in Its Present State
The painting at The J. Paul Getty Museum
Video narrative about the painting from The Getty Museum.
Rembrandt van Rijn, An Old Man in Military Costume, 1630-31.
Image courtesy of The J. Paul Getty Trust.
Conventional X-Ray Radiography
The painting was first investigated by conventional X-ray radiography and an underlying portrait of a young man was discovered beneath the visible image. The quality of the x-ray image was not sufficient to discern any details of the underlying portrait. It should be noted that Rembrandt rotated the canvas 180 degrees before he overpainted the old image with the ‘An Old Man in Military Costume‘.
X-radiographs of An Old Man in Military Costume. The x-radiograph at right is inverted to better show the hidden painting.
Image courtesy of The J. Paul Getty Trust.
NAAR and XRF-Investigations (1,2)
The latest investigation by Trentelman et al. (2) employing both X-ray fluorescence (XRF) and neutron activation autoradiography (NAAR) brought a considerable improvement in image quality and in the identification of pigments as well. The NAAR images showed improved detail in the face and the cloak of the young man and the XRF data made it possible to roughly identify the pigments used by Rembrandt in the underlying painting (1).
The NAAR radiograph shown below is a composite image of 6 plates exposed after 8 days and 9 hours after removing it from the reactor with an exposure time of 2 days and 12 hours. It is rotated 180 degrees to better show the portrait of the young man.
Rembrandt, An Old Man in Military Costume, 1630-31. Inverted NAAR Autoradiograph.
Image courtesy of The J. Paul Getty Trust.
Digital Reconstruction of the Underlying Portrait
The results of the entire investigation using both the NAAR and XRF methods could then be converted into a colour digital reconstruction of the underlying portrait of the young man. Comparing this image to the conventional x-ray images above shows the impressive extent of the technical development of the scientific investigation methods in the last decades.
Digital reconstruction of the underlying portrait of the young man.
Image courtesy of The J. Paul Getty Trust.
References
(1) Annelisa Stephan, A Hidden Rembrandt Has Been Digitally Reconstructed in Color, IRIS – The online magazine of The Getty, 1 September 2015.
(2) Karen Trentelman, Koen Janssens, Geert van der Snickt, Yvonne Szafran, Anne T. Woollett, Joris Dik, Rembrandt’s An Old Man in Military Costume: the underlying image re-examined, Applied Physics A, November 2015, Volume 121, Issue 3, pp 801-811. Available as pdf.
Rembrandt, Susanna and the Elders, 1635-47
The Painting in Its Present State
The painting is in the collection of the Gemäldegalerie Berlin. The entry in The Rembrandt Database contains a very detailed report (by C. Laurenze-Landsberg, in German) on the results of all investigations. There is a long section on the results of the neutron activation analysis containing high-resolution images of five autoradiographs of this painting.
The authors of the investigation employed both neutron activation autoradiography and x-ray fluorescence (XRF) to compare both methods and establish their advantages and disadvantages.
Neutron Activation Radiography Investigation
Five consecutive film sets were exposed by C. Laurenze-Landsberg at the Gemäldegalerie and the scientists at the Helmholtz-Zentrum in Berlin (1). Three of these autoradiographs are shown below courtesy of the authors. All images © Gemäldegalerie, Staatliche Museen Berlin, and Helmholtz-Zentrum Berlin.
Neutron autoradiograph # 2: the exposition was started after 6h 45 m after the end of the activation and lasted 17 h. The deep black areas were caused by the radiation of the copper isotope 64Cu contained in blue pigments such as azurite. (It is known that no green copper-containing pigments were employed by Rembrandt in this painting.) Blackening caused by the arsenic isotope 76As contained in the blue pigment smalt can also be discerned. Small dark areas caused by the radiation of the mercury isotope 197Hg contained in the red pigment vermilion, the antimony isotope 122Sb contained in naples yellow, and the tin isotope 121Sn contained in lead-tin yellow are also present.
Neutron autoradiograph # 3: the exposition was started after 1 day and 6 hours after the end of the activation and lasted 1 day and 18 h. During the beginning part of the exposition, the copper isotope is still radiating with around 1/8 of its original intensity. The blackening caused by the radiation of this isotope is almost as strong as in the second autoradiograph due to the long exposure. The arsenic isotope in smalt is still radiating with approximately 1/4 of its original intensity so the blackening accumulated during the long exposure in this autoradiograph is even stronger than in the second one above
Neutron autoradiograph # 5: the exposition was started after 12 days after the end of the activation and lasted 46 days. The radiation from the copper and arsenic isotopes was already completely decayed at the time of this exposition. This film registered the radiation from the phosphorus isotope 32P in bone black, the mercury isotopes 197Hg and 203Hg in vermilion, the cobalt isotope 60Co in smalt and finally the antimony isotopes 122Sb and 124Sb in Naples yellow.
New Insights From the Investigation
The autoradiographs of this painting brought interesting insights into Rembrandt’s working process. The painting contains a large number of pentimenti which could be seen in the x-ray radiographs but many more details came to light from the evaluation of the neutron autoradiography and the single-element maps from the x-ray fluorescence investigation (1). One example of the change of the original composition is the arm and hand of the Elder in the foreground. Originally he was touching Susanna’s naked body while in the latest version his hand rests on Susanna’s towel.
It was also discovered that the painting was grossly overpainted by Sir Joshua Reynolds (1723-1792) long after Rembrandt’s death.
References
(1) Matthias Alfeld, Claudia Laurenze-Landsberg, Andrea Denker, Koen Janssens, Petria Noble, Neutron activation autoradiography and scanning macro-XRF of Rembrandt van Rijn’s Susanna and the Elders (Gemäldegalerie Berlin): a comparison of two methods for imaging of historical paintings with elemental contrast. Appl. Phys. A (2015) 119:795–805, DOI 10.1007/s00339-015-9081-8.
(2) H. Bevers, K. Kleinert, C. Laurenze-Landsberg, Katalog zur Ausstellung „Rembrandts Berliner Susanna und die beiden Alten, Die Schaffung eines Meisterwerkes“, Leipzig 2015.
(3) Ernst Van De Wetering, Rembrandt — Susanna and the Elders, Berlin, Staatliche Museen zu Berlin, Gemäldegalerie, Cat. No. 828 E, in A Corpus of Rembrandt Paintings, Volume 5 of the series, Stichting Foundation, Rembrandt Research Project, pp 325-342.
Johannes Vermeer, Woman with a Pearl Necklace, 1662-65
The Painting in Its Present State
Neutron Activation Radiography Investigation
Five consecutive film sets were exposed by C. Laurenze-Landsberg at the Gemäldegalerie and the scientists at the Helmholtz-Zentrum in Berlin. Three of these autoradiographs are shown below courtesy of the authors (1). All images © Gemäldegalerie, Staatliche Museen Berlin and Helmholtz-Zentrum Berlin.
Neutron autoradiograph # 1: exposition was started 30 m after the end of the activation and lasted 1 hour and 30 min. The very fast decaying isotope of manganese 56Mn contained in the brown pigment umber caused the darkening in the areas of the chair and in the wooden frame. The only a little slower decaying copper isotope 64Cu contained in green and blue pigments (azurite, malachite, and verdigris) has darkened the areas of the tablecloth and the vase.
Neutron autoradiograph # 3: the exposition started 1 day after the end of the activation and lasted 2 days. We can observe blackening of the film caused by the arsenic isotope 76As contained in the pigment smalt in the vase, as well as blackening caused by the two isotopes of mercury 197Hg and 203Hg contained in vermilion in the hair ribbon.
Neutron autoradiograph # 5: the exposition was started 9 days after the end of the activation and lasted 42 days. These films registered the radiation from the phosphorus isotope 32P in bone black and the mercury isotopes 197Hg and 203Hg in vermilion.
New Insights From the Investigation
The changes in the composition can be identified in this autoradiograph: the map on the back wall, the lute on the chair, and the tiles on the floor are not visible in the painting in its present state and were overpainted by Vermeer.
References
(1) C. Laurenze-Landsberg, “Neutron-Autoradiography of Two Paintings by Jan Vermeer in the Gemäldegalerie Berlin”, W. Lefèvre, Inside the Camera Obscura – Optics and Art under the Spell of the Projected Image, Reprint 333, Berlin 2007, S. 211-225.
Further Reading
References
(1) A. Denker, C. Laurenze-Landsberg, K. Kleinert, B. Schröder-Smeibidl, Paintings Reveal Their Secrets: Neutron Autoradiography Allows the Visualization of Hidden Layers, in ‘Neutron Methods for Archaeology and Cultural Heritage’, Eds. N. Kardjilov, G. Festa, Springer International Publishing, Cham, 2017, p. 41, DOI: 10.1007/978-3-319-33163-8_3
(2) Sayre, Edward V.; Lechtman, Heather N., Neutron activation autoradiography of oil paintings, Studies in conservation, Volume 13, Number 4, p.161-185 (1968).
(3) A. Kalicki, E. Panczyk, L. Rowinska, B. Sartowska, L. Walis, K. Pytel, B. Pytel, A. Koziel, L. Dabkowski, M. Wierzchnicka, L. Strzalkowski, T. Ostrowski, Neutron autoradiography: working-out method and application in investigations of test paintings, Radiation Measurements, Volume 34, Issues 1–6, June 2001, Pages 567–569, Proceedings of the 20th International Conference on Nuclear Track s in Solids.
(4) Maurice J. Cotter, Neutron Activation Analysis of Paintings: Autoradiography following neutron irradiation provides information for authentication and restoration and reveals the existence of underpaintings undetected by other means, American Scientist, Vol. 69, No. 1 (January-February 1981), pp. 17-27.
(5) E. Pańczyk, K. Pytel, A. Kalicki, L. Rowińska, B. Sartowska and L. Waliś, Neutron-induced autoradiography in the study of oil paintings by Tintoretto, Marieschi and Bellotto, MRS Proceedings / Volume 712 / 2002, 2001 Material Research Society (MRS) Fall Meeting.
(6) Kathleen K. Taylor, Maurice J. Cotter and Edward V. Sayre, Neutron Activation Autoradiography as a Technique for Conservation Examination of Paintings, Bulletin of the American Institute for Conservation of Historic and Artistic Works, Vol. 15, No. 2 (Summer, 1975), pp. 93-102. DOI: 10.2307/3179299.
(7) C.-O. Fischer, C. Laurenze-Landsberg W. Leuther und K. Slusallek, Neutronenautoradiographie. Tiefenanalyse des Bildes, Physikalische Grundlagen, Technische Durchführung, in J. Kelch (Hrsg.), Bilder im Blickpunkt: Der Mann mit dem Goldhelm, Berlin 1986, S. 38-47.
(8) Maryan Wynn Ainsworth, Art and Autoradiography: Insights Into the Genesis of Paintings by Rembrandt, Van Dyck and Vermeer, Metropolitan Museum of Art, 1982.