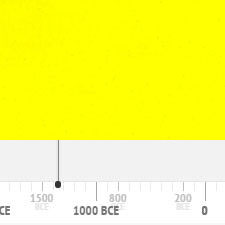
Orpiment
Natural mineral pigmentComposition and Properties of Orpiment
Orpiment is yellow arsenic sulfide with the formula As2S3. It occurs naturally as a mineral and can also be prepared artificially.
The pigment is not very stable and decomposes slowly in contact with water, it is, however, more stable when dry. It is considered fairly lightfast but it can in some cases darken considerably and it is not compatible with lead and copper pigments.
Names
Alternative names
King’s yellow, yellow arsenic sulfide
Color Index
PY 39, CI 77085 and 77086
Word origin
From Latin auripigmentum, from aurum “gold” + pigmentum “coloring matter, pigment, paint,” from stem of pingere “to color, paint,”
Auripigment
German
Orpiment
French
Orpimento
Italian
Oropimento
Spanish
Preparation
Attention: Arsenic compounds are toxic and should not be handled by people not trained to do so.
The mineral has to be broken, ground and purified.
Preparation of artificial orpiment
The pigment can be prepared by heating and subsequent sublimation of a mixture of sulfur and arsenic oxides.
Video: 'Arsenic-based pigments' by Hegelrast
History of Use
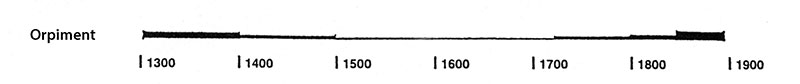
It has been in use since antiquity until about the nineteenth century. The following graph gives the frequency of its use in the paintings of the Schack Collection in the Bavarian State Art Collections in Munich (1).
References
(1) Kühn, H., Die Pigmente in den Gemälden der Schack-Galerie, in: Bayerische Staatsgemäldesammlungen (Ed.) Schack-Galerie (Gemäldekataloge Bd. II), München 1969.
Examples of use
Paolo Veronese, Allegory of Love – Happy Union, last quarter of the 16th century

Tintoretto, Portrait of Vincenzo Morosini, about 1575-80

7 Orange spots on the golden embroidery of the sash: realgar
8 Yellow parts of the embroidery of the sash: orpiment and realgar

Identification
Fiber optics reflectance spectra (FORS)
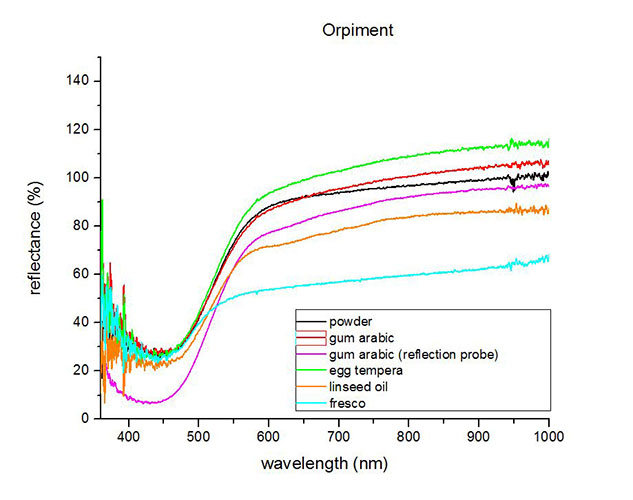
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
Infrared Spectrum
1. IR Spectrum of orpiment in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia.
2. IR Spectrum of orpiment in linseed oil by S. Vahur, Database of ATR-IR spectra of materials related to paints and coatings, University of Tartu, Estonia.
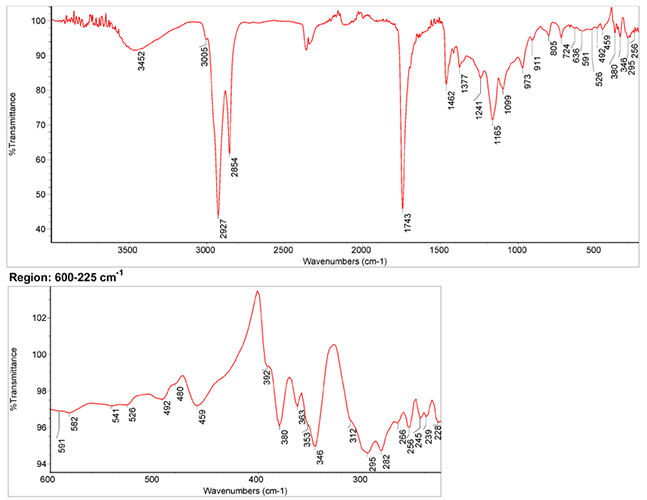
Raman Spectrum
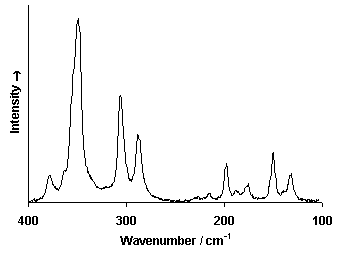
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
References
(1) Orpiment, The Conservation and Art Materials Encyclopedia Online (CAMEO), Forbes Pigment Database. Contains XRD, SEM, XRF and EDF spectra and a SEM image of orpiment.

Microphotograph
image © Volker Emrath
Further Reading
References
(1) Fitzhugh, E.W., Orpiment and Realgar, in Artists’ Pigments, A Handbook of Their History and Characteristics, Vol 3: E.W. Fitzhugh (Ed.) Oxford University Press 1997, p. 47 – 80. Available as pdf from the National Gallery of Art.
(2) Wallert, A. Orpiment und Realgar, Maltechnik-Restauro, 90, 1984, p. 45.
(3) Carolin Rötter, Auripigment, Restauro, 6 2003, 408-413.
(4) Orpiment, CAMEO Materials Database, Forbes Pigment Database
(5) Rötter, C., Grundmann, G., Richter, M., Loon, A. van, Keune, K., Boersma, A., & Rapp, K., Auripigment / Orpiment. Studien zu dem Mineral und den künstlichen Produkten / Studies on the mineral and the artificial products. Verlag Anton Siegel, 2007.
(6) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 42-43.