Prussian Blue
Artificial inorganic pigmentComposition and Properties of Prussian Blue
Prussian blue is an inorganic complex salt containing two differently charged iron ions Fe2+ and Fe3+ and the negatively charged hexacyanoferrate ions [Fe(CN)6]4–. The overall formula is usually written as Fe4[Fe(CN)6]3· x H2O. There are also variants containing other positive ions such as potassium K+ or sodium Na+.
The pigment is decomposed by dilute alkalis to form brown iron hydroxide, but it is resistant against dilute mineral acids and withstands temperatures up to 140 °C. It is compatible with many pigments that are not alkaline, so it should not be mixed with lead white and calcium carbonate. The reports on the stability of this pigment vary and its lightfastness depends on several factors. In pure form, it is a permanent pigment but its lightfastness diminishes when mixed with other pigments.
It is compatible with many pigments that are not alkaline, so it should not be mixed with lead white and calcium carbonate.
Art Teachers' Materials
Richly illustrated presentation on the properties, preparation, identification and use of this pigment
Names
Alternative names
Milori blue, iron blue, Turnbull’s blue
Color Index
PB 27, CI 77510
Word origin
From Prussia, the country in northern Germany
Berlinerblau
German
bleu prussien
French
azzuro di Prussia, azzuro di Berlino
Italian
azul de Prussia, azul de Berlin
Spanish
Preparation of Prussian Blue
The pigment can be prepared by a reaction of ferric chloride FeCl3 with potassium ferrocyanide K4[Fe(CN)6] in solution.
Video: 'Pigment Synthesis' by The Alchemical Arts
Video: 'Making the Pigment' by NileRed
History of Use
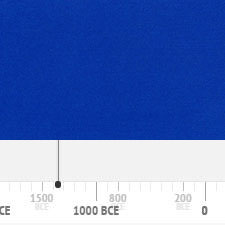
It is the oldest modern synthetic pigment that has been in use since its discovery in 1704 until the present day. The following graph gives the frequency of its use in the paintings of the Schack Collection in the Bavarian State Art Collections in Munich (1).

Examples of use
Vincente Palmaroli, María de los Dolores Collado y Echagüe, duquesa de Bailén, ca 1870

References
(1) Kühn, H., Die Pigmente in den Gemälden der Schack-Galerie, in: Bayerische Staatsgemäldesammlungen (Ed.) Schack-Galerie (Gemäldekataloge Bd. II), München 1969.
(2) Alexander Kraft, On the discovery and history of Prussian blue, Bull. Hist. Chem. 33, Number 2 (2008)
(3) Bartoll, J., The early use of Prussian blue in paintings, 9th International Conference on NDT of Art, Jerusalem Israel, 25-30 May 2008.
(4) L.J.M. Coleby M.A. M.Sc. Ph.D. A history of Prussian blue, Annals of Science, 4:2, 1939, 206-211, DOI: 10.1080/00033793900201211
Identification
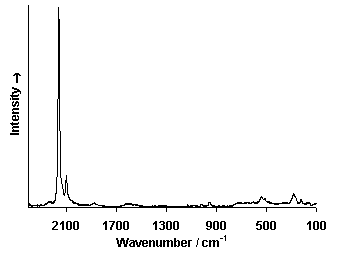
Fiber Optics Reflectance Spectrum (FORS)
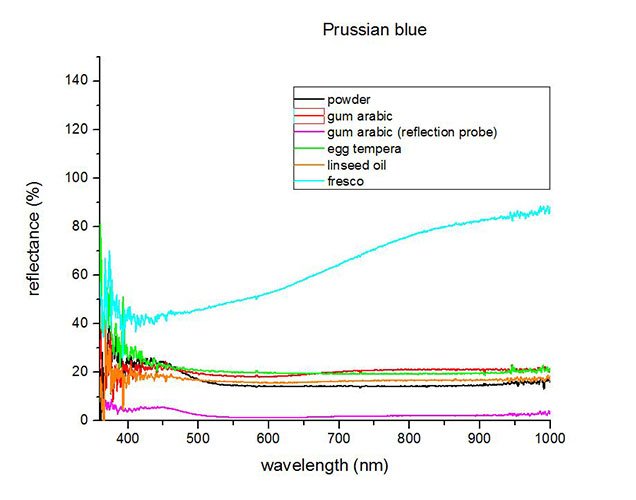
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
Infrared Spectrum
- The spectrum of the pigment in linseed oil by S. Vahur, Database of ATR-IR spectra of materials related to paints and coatings, University of Tartu, Estonia
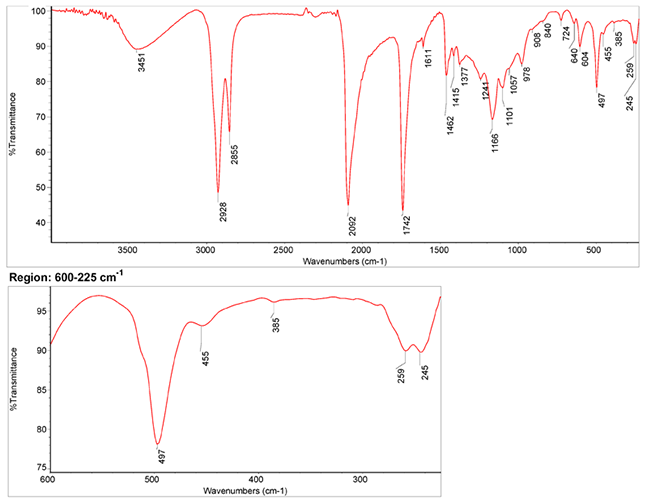
2. IR-Spectrum of the pigment in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia
Raman Spectrum
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
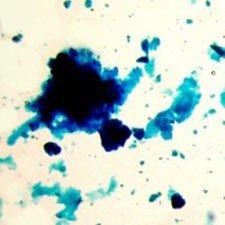
Microphotograph
image © Volker Emrath
Further Reading
References
(1) Barbara H. Berrie, Prussian Blue, in Artists’ Pigments, A Handbook of Their History and Characteristics, Vol 3: E.W. Fitzhugh (Ed.) Oxford University Press 1997, p. 191 – 217. Available as pdf from the National Gallery of Art.
(2) Kirby, J. Saunders, D. Fading and Colour Change of Prussian Blue: Methods of Manufacture and the Influence of Extenders. National Gallery Technical Bulletin, 25, 2004, 73–99.
(3) Ludi, A. Prussian blue, an inorganic evergreen. Journal of Chemical Education, 58 (12), 1981, 1013. doi:10.1021/ed058p1013.
(4) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 88-89.