Titanium Dioxide White
Synthetic inorganic pigmentComposition and Properties of Titanium Dioxide White
Titanium dioxide white consists of titanium dioxide TiO2.

Pigment

Painted swatch
Titanium dioxide can be found in nature in several different mineral forms. The forms which can be utilized as pigments are rutile and anatase

Rutile crystals embedded in quartz
Rob Lavinsky, iRocks.com – CC-BY-SA-3.0

Mineral-Anatase
Image: Didier Descouens
Video 'Titanium White Oil Paints' by Nelson Ferreira
Video 'Titanium & Zinc White' by CITY STATIONERY GROUP SAL
Names
Alternative names
Titanox
Color Index
PW 6, CI 77891
Word origin
Titanium: from Latin titan, from Greek titan, member of a mythological race of giants (originally six sons and six daughters of Gaia and Uranus) who were overthrown by Zeus and the other gods. The name is perhaps from tito “sun, day,”
From Online Etymology Dictionary
Titanweiss
German
Oxyde de titane
French
Bianco di titanio
Italian
Dióxido de titanio
Spanish
Preparation
Titanium-containing ores are converted into pure titanium oxide. The two main methods for this transformation are the sulfate process and the chloride process (1).
Sulfate Process
The titanium ore ilmenite (FeTiO3) is treated with sulfuric acid:
FeTiO3 + 2H2SO4 → FeSO4 + TiOSO4 + 2H2O
The titanium oxosulfate is then separated and converted to hydrated titanium dioxide (TiO2 • n H2O) by heating. In the last stage of the production process, the water is removed by even stronger heating.
Chloride Process
The raw material for this process must contain at least 70% rutile. Titanium dioxide is reduced with carbon and then oxidized again
with chlorine.
TiO2 + C → Ti + CO2
Ti + 2Cl2 → TiCl4
Titanium chloride is liquid at room temperature and can be distilled off and converted to TiO2 by burning in oxygen. Chlorine produced in this reaction can be recovered and reused.
TiCl4 + O2 → TiO2 + 2Cl2
References
(1) Corina E. Rogge & Julie Arslanoglu, Distinguishing manufacturing practices for titanium white pigments: New Raman markers for dating commercial oil-based paints, Studies in Conservation, Volume 61, 2016 – Issue sup2: LA Congress Preprints Modern Art, Pages 324-326.
(2) Foord von Bichowsky, Titanium White – A New Method for its Preparation, Ind. Eng. Chem., 1929, 21 (11), pp 1061–1063
DOI: 10.1021/ie50239a021
Video: 'Mining and Milling Ore for Titanium Dioxide' by National Lead Company, 1954
History of Use
Titanium dioxide white has been used by a multitude of painters after its first production date between 1910 and 1920.
References
(1) van Driel, B.A., van den Berg, K.J., Gerretzen, J. et al. The white of the 20th century: an explorative survey into Dutch modern art collections. Herit Sci 6, 16 (2018). https://doi.org/10.1186/s40494-018-0183-4
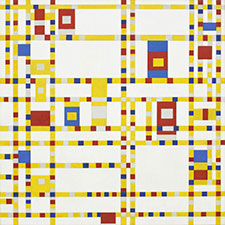
Examples of use
Franz Kline, Chief, 1950

Identification
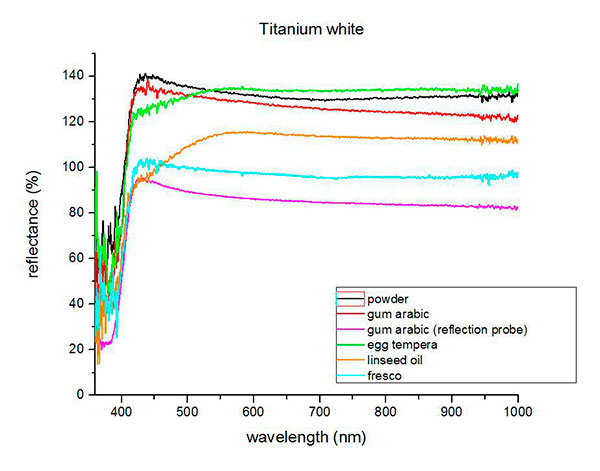
Fiber optics reflectance spectra (FORS)
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
(1) Mauro Bacci, Marcello Picollo, Giorgio Trumpy, Masahiko Tsukada and Diane Kunzelman, Non-Invasive Identification of White Pigments on 20th-Century Oil Paintings by Using Fiber Optic Reflectance Spectroscopy, Journal of the American Institute for Conservation, Vol. 46, No. 1 (Spring, 2007), pp. 27-37.
IR Spectrum
1. IR Spectrum of titanium dioxide white in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia.
2. IR Spectrum of titanium dioxide white in linseed oil by S. Vahur, Database of ATR-IR spectra of materials related to paints and coatings, University of Tartu, Estonia.
Raman Spectrum
X-Ray Fluorescence Spektrum (XRF)
References
(1) Fortunato, D. F. and G. (n.d.). Tracing White: A Study of Lead White Pigments found in Seventeenth-Century Paintings using High Precision Lead Isotope Abundance Ratios.
(2) V. Gonzalez, T. Calligaro, G. Walleza, M. Eveno, K. Toussaint, M. Menu, Composition and microstructure of the lead white pigment in Masters paintings using HR Synchrotron XRD, Microchemical Journal, Volume 125, March 2016, Pages 43-49
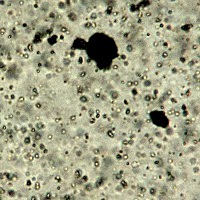
Microphotograph
image © Volker Emrath
Further Reading
References
(1) Marilyn Laver, Titanium Dioxide Whites, in Artists’ Pigments, A Handbook of Their History and Characteristics, Vol 3: E.W. Fitzhugh (Ed.) Oxford University Press 1997, p. 295 – 355. Available as pdf from the National Gallery of Art.
(2) Corina E. Rogge & Julie Arslanoglu, Distinguishing manufacturing practices for titanium white pigments: New Raman markers for dating commercial oil-based paints, Studies in Conservation, Volume 61, 2016 – Issue sup2: LA Congress Preprints Modern Art, Pages 324-326.
(3) Mauro Bacci, Marcello Picollo, Giorgio Trumpy, Masahiko Tsukada and Diane Kunzelman, Non-Invasive Identification of White Pigments on 20th-Century Oil Paintings by Using Fiber Optic Reflectance Spectroscopy, Journal of the American Institute for Conservation, Vol. 46, No. 1 (Spring, 2007), pp. 27-37.
S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, p. 119.