Azurite
Natural inorganic pigmentComposition and Properties of Azurite
Azurite is basic copper (II)-carbonate: 2 CuCO3·Cu(OH)2 forming bright blue crystals. The pigment has been prepared either from naturally occurring mineral or produced synthetically. The artificial variety is known under the name blue verditer.
It is lightfast and stable in contact with the atmosphere and withstands higher temperatures up to its decomposition at approximately 300° C. It is resistant to cold alkalis but is dissolved by dilute acids as all carbonates are. Its color can change to green because of its transformation to malachite.
Text in Pliny's "Natural History"
Pliny the Elder, The Natural History, Book 35, Chapter 28
Pliny describes azurite under the name of lapis armenius (Armenian stone).
“Armenia sends us the colouring substance which is known to us by its name. This also is a mineral, which admits of being dyed, like chrysocolla, and is best when it most closely resembles that substance, the colour being pretty much that of cæruleum. In former times it was sold at thirty sesterces per pound; but there has been found of late in the Spanish provinces a sand which admits of a similar preparation, and consequently armenium has come to be sold so low as at six denarii per pound. It differs from cæruleum in a certain degree of whiteness, which causes the colour it yields to be thinner in comparison. The only use made of it in medicine is for the purpose of giving nourishment to the hair, that of the eyelids in particular.”
The Natural History. Pliny the Elder. John Bostock, M.D., F.R.S. H.T. Riley, Esq., B.A. London. Taylor and Francis, Red Lion Court, Fleet Street. 1855. Available online in Perseus Digital Library.
Video: "A Study in Azurite" by Kodner Galleries

Pigment

Painted swatch
Names of Azurite
Alternative names
Mountain blue, lapis armenius,
Color Index
PB 30, CI 77420
Word origin
From Arabic al = the and lāzaward from Persian lāžward = lapis lazuli
Azurit
German
Azurite
French
Azzurite
Italian
Azurita
Spanish
Preparation of Azurite
The naturally occurring mineral is broken, ground, purified by levigation (milling with water), and sieved. Azurite can also be prepared artificially and is then called blue verditer.
Video: "Grinding Azurite Pigment" by Ronnie Cruwys

Mineral from Australia
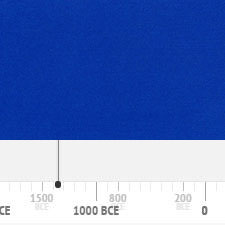
History of Use of Azurite
Azurite has been in use since antiquity until about the beginning of the 18th century. The following graph gives the frequency of its use in the paintings of the Schack Collection in the Bavarian State Art Collections in Munich (1).

References
(1) Kühn, H., Die Pigmente in den Gemälden der Schack-Galerie, in: Bayerische Staatsgemäldesammlungen (Ed.) Schack-Galerie (Gemäldekataloge Bd. II), München 1969.
(2) Mira S. de Roo, The Trade in Blue During the 17th Century: An Examination of Western European Pigment Trade in Azurite, Indigo, Lapis Lazuli, and Smalt During the 17th Century Through Works in the National Gallery, London, Master of Philosophy, University of Glasgow, 2004.
Examples of use
Raphael, Procession to Calvary, 1504-05
Identification of Azurite
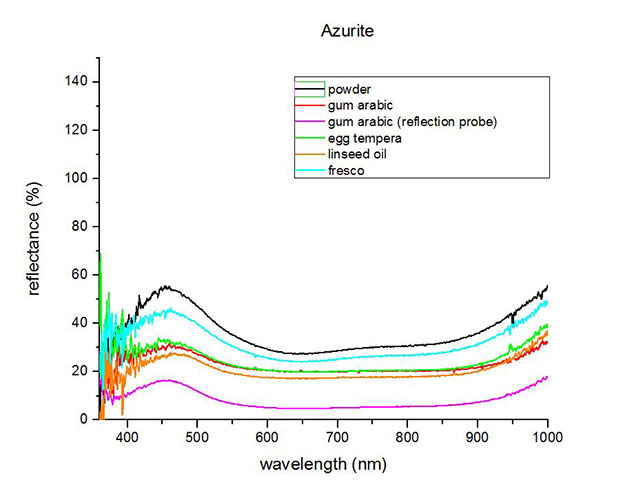
Fiber Optics Reflectance Spectrum (FORS)
FORS spectrum by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
Infrared Spectrum
- IR Spectrum of azurite in linseed oil by S. Vahur, Database of ATR-IR spectra of materials related to paints and coatings, University of Tartu, Estonia
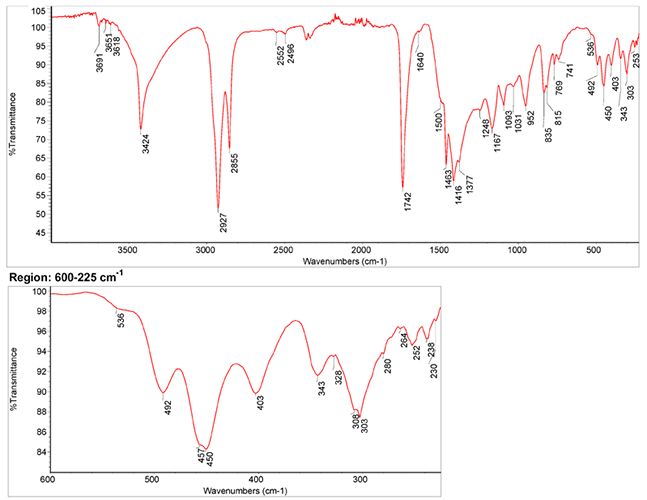
2. IR-Spectrum in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia
Raman Spectrum
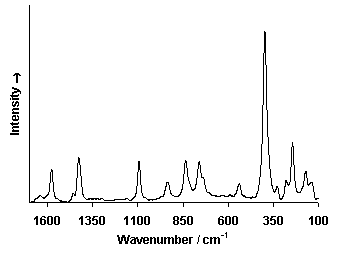
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.

Microphotograph
image © Volker Emrath
Further Reading About Azurite
References
(1) Gettens, R.J. and Fitzhugh, E.W., Azurite and Blue Verditer, in Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 2: A. Roy (Ed.) Oxford University Press 1993, p. 23-35. Available as pdf from the National Gallery of Art.
(2) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 48-49.