Lead-Tin Yellow
Artificial inorganic pigmentComposition and Properties of Lead-Tin Yellow
Lead-tin yellow is known in two different forms. The first and more frequently used is called type I and is a mixed oxide of both elements tin and lead with the chemical formula of Pb2SnO4. Type II possibly contains traces of silica and also pure tin oxide.
Lead-tin yellow is chemically quite stable under normal conditions and is also resistant to high temperatures. It is also resistant to acidic and alkaline solutions and can thus be employed in fresco. The light steadfastness of the pigment is very high but it is blackened by contact with sulfides or gaseous hydrogen sulfide.

Pigment

Painted swatch
Names of Lead-Tin Yellow
Alternative names
Giallolino, giallorino, massicot, masticot
Color Index
CI 77629
Word origin
Lead: Old English lead “lead, leaden vessel,” from West Germanic *lauda– a word of uncertain origin.
Tin: Old English tin, from Proto-Germanic *tinom, of unknown origin
Blei-Zinn Gelb
German
Jaune de plomb étain
French
Giallo di piombo-stagno
Italian
Amarillo de plomo-estano
Spanish
Preparation of Lead-Tin Yellow
Attention: All lead salts are highly toxic chemicals and should not be used by people not trained to handle them.
The pigment can be prepared by heating a mixture of any lead oxide and tin dioxide in an oven.
Preparation of Lead-Tin Yellow in the Lab
A mixture of 3 parts of any oxide of lead such as lead monoxide (PbO), dioxide (PbO2) or minium (Pb3O4) and 1 part of tin dioxide (SnO2) is homogenized in a porcelain mortar and transferred into a crucible.


The crucible is then placed in an oven and heated to a temperature of 650 to 700° C. The reaction is complete after about 30 minutes.


References
(1) C. Pelosi at al., Artificial Yellow Pigments: Production and Spectroscopic Characterization, e-Preservation Science, 2010, 7, 108-115.
(2) Robin J. H. Clark, Lucas Cridland, Benson M. Kariuki, Kenneth D. M. Harris and Robert Withnall, Synthesis, structural characterization and Raman spectroscopy of the inorganic pigments lead-tin yellow types I and II and lead antimonate yellow: their identification on medieval paintings and manuscripts. J. Chem. Soc., Dalton Trans., 1995, 2577-2582. DOI: 10.1039/DT9950002577.
(3) Agresti G., Baraldi P., Pelosi C. and Santamaria U.,Yellow pigments based on lead, tin, and antimony: Ancient recipes, synthesis, characterization, and hue choice in artworks, Color Research and Application 41 (3) 2016. https://doi.org/10.1002/col.22026
History of Use
The first occurrences of lead-tin yellow in paintings date from around 1300, the most frequent use followed in the fifteenth, sixteenth and seventeen centuries. The use of this pigment seems to have ceased around 1750. Interestingly enough, it was rediscovered in 1941 (1). The following graph gives the frequency of its use in the paintings of the Schack Collection in the Bavarian State Art Collections in Munich (2).

References
(1) H. Kühn, Lead-Tin Yellow, in Artists’ Pigments, a Handbook of their History and Characteristics, vol. 2, National Gallery of Art, Washington, 1993, p. 85
(2) Kühn, H., Die Pigmente in den Gemälden der Schack-Galerie, in: Bayerische Staatsgemäldesammlungen (Ed.) Schack-Galerie (Gemäldekataloge Bd. II), München 1969.
Examples of use
Anthony van Dyck, ‘Lady Thimbelby and her Sister Dorothy, Viscountess Andover‘, ca 1637

8 Viscountess’s golden-yellow dress: lead-tin yellow. The darker and more golden tones contain deep yellow ochre with white and a little crystalline red ochre (haematite). The more orange tones of the sleeve are painted with a higher proportion of haematite, as well as lead-tin yellow, yellow ochre and red lake.

Rembrandt van Rijn, Belshazzar’s Feast, about 1636-38

The richly brocaded cloak of Belshazzar and the inscription on the wall are painted in pure lead-tin-yellow.
Johannes Vermeer, The Milkmaid, 1657-58

The yellow upper part of the tunic is painted in lead-tin-yellow and contrasts nicely with the blue skirt. The lighter parts of the sleeve are painted in lead-tin-yellow mixed with natural ultramarine, while the darker parts contain ultramarine mixed with lead white and are overglazed with ultramarine. In the blue-gray overturned sleeve Vermeer painted first a ground layer of ochre which he then overpainted by a mixture of ultramarine and lead white.

Identification
Microanalytical methods
Hradil, David, Tomáš Grygar, Janka Hradilová, Petr Bezdička, Veronika Grűnwaldová, Igor Fogaš, and Costanza Miliani. “Microanalytical Identification of Pb-Sb-Sn Yellow Pigment in Historical European Paintings and Its Differentiation from Lead Tin and Naples Yellows.” Journal of Cultural Heritage 8, no. 4 (2007): 377–86. doi:10.1016/J.CULHER.2007.07.001.
Fiber Optics Reflectance Spectroscopy (FORS)
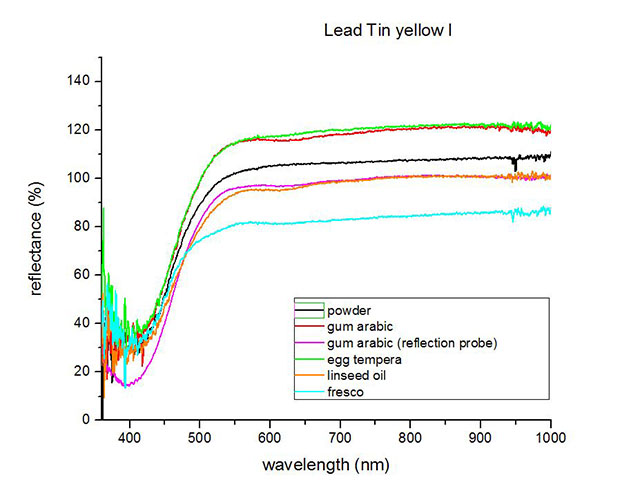
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
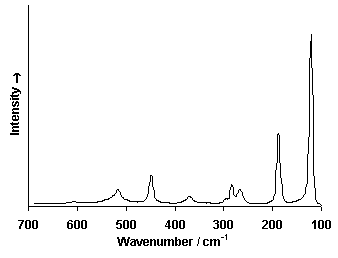
Raman Spectrum
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
(1) Robin J. H. Clark, Lucas Cridland, Benson M. Kariuki, Kenneth D. M. Harris and Robert Withnall, Synthesis, structural characterization and Raman spectroscopy of the inorganic pigments lead-tin yellow types I and II and lead antimonate yellow: their identification on medieval paintings and manuscripts. J. Chem. Soc., Dalton Trans., 1995, 2577-2582. DOI: 10.1039/DT9950002577.
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum of lead-tin yellow type I in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
XRF Spectrum of lead-tin yellow type II in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
NMR Spectroscopy
Solid-state NMR investigation of lead-tin-yellow pigment: Metropolitan Museum of Art and the University of Delaware.
Microphotograph
image © Volker Emrath
Further Reading
References
(1) H. Kühn, Lead-Tin Yellow, Studies in Conservation Vol. 13, No. 1 (Feb 1968), pp. 7-33, Reprinted with revisions in Artists’ Pigments, a Handbook of their History and Characteristics, vol. 2, National Gallery of Art, Washington, 1993). Available as pdf from the National Gallery of Art.
(2) Kühn, H., Blei-Zinn-Gelb und seine Verwendung in der Malerei, Farbe und Lack, 73, 1967, s. 938-949
(3) Robin J. H. Clark, Lucas Cridland, Benson M. Kariuki, Kenneth D. M. Harris and Robert Withnall, Synthesis, structural characterization and Raman spectroscopy of the inorganic pigments lead-tin yellow types I and II and lead antimonate yellow: their identification on medieval paintings and manuscripts. J. Chem. Soc., Dalton Trans., 1995, 2577-2582. DOI: 10.1039/DT9950002577.
(4) Nicholas John Eastaugh, Lead-tin yellow: its history, manufacture, colour and structure, University of London, 1988.
(5) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 80-81 and 360-367.