Malachite
Natural inorganic pigmentComposition and Properties of Malachite
Malachite is a basic copper carbonate with the chemical formula of CuCO3 · Cu(OH)2.
It is not stable at high temperatures, its decomposition begins at about 200 °C. Malachite, as all carbonates, is dissolved by even dilute acids but is unaffected by cold solutions of hydroxides.
Video: 'Malachite in art | Why this paint didn’t last 500 years' by National Gallery London
David Peggie from the Scientific department discusses Giovanni Bellini’s use of the pigment malachite, in his painting ‘The Agony in the Garden‘.

Pigment

Painted swatch
Names
Alternative names
Mountain green, Olympian green, Hungarian green, copper green
Color Index
PG 39, CI 77492
Word origin
From French malachite, ultimately from Greek malachitis (lithos) “mallow (stone),” from malakhe “mallow”; the mineral traditionally so called from the resemblance of its color to that of the leaves of the mallow plant.
From Online Etymology Dictionary
Malachit
German
Malachite
French
Malachito
Italian
Malaquita
Spanish
Preparation
The pigment can be prepared from the mineral malachite by grounding, washing and levigating the raw material.
Malachite can also be prepared in the laboratory by a reaction of copper (II) sulfate and sodium carbonate. The artificial form is sometimes called green verditer.
Preparation of Malachite in the Lab
12,5 g hydrated copper (II) sulfate CuSO4.5H2O is dissolved in 50 mL deionized water. A solution of 5,8 g Na2CO3 in 55 mL deionized water is then added slowly to the first solution while mixing vigorously.

After adding about 40 mL of the sodium carbonate solution the mixture starts to liberate gaseous carbon dioxide.

The resulting mixture is then left standing for one to two days at a temperature of 5 to 10 °C. The precipitate is then washed twice with deionized water, filtered and dried.

Video: 'Making Basic Copper Carbonate' by NileRed
History of Use
Malachite has been used in ancient Egypt, but despite its abundance, it was seldom used in European oil painting. The following graph gives the frequency of its use in the paintings of the Schack Collection in the Bavarian State Art Collections in Munich (1).
An extensive listing of occurrences of malachite in paintings in several historical periods can be found in the blog post ‘Pigment: The unusual green of Malachite‘ by The Eclectic Light Company.
References
(1) Kühn, H., Die Pigmente in den Gemälden der Schack-Galerie, in: Bayerische Staatsgemäldesammlungen (Ed.) Schack-Galerie (Gemäldekataloge Bd. II), München 1969.
Examples of use
Raphael, Sistine Madonna, 1512
The green drapery in the upper part of the painting: lead white, charcoal black, orpiment and malachite

Tintoretto, St George and the Dragon, about 1555
5 Green landscape: malachite and smaller amounts of verdigris.
6 Yellow-green parts of the landscape: malachite with smaller amounts of verdigris mixed with lead-tin-yellow.

Identification
Fiber Optics Reflectance Spectra (FORS)
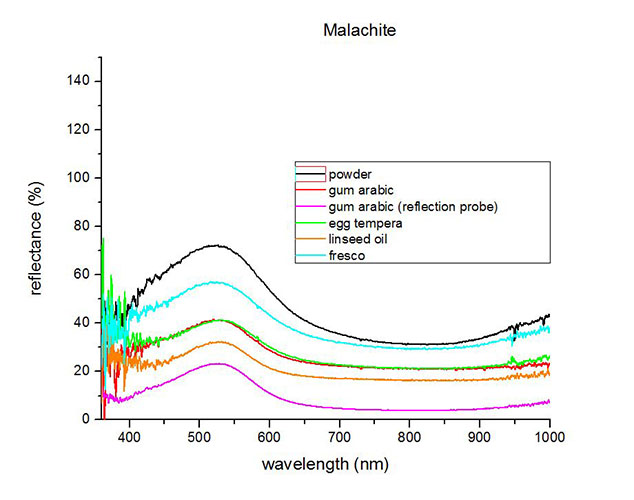
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
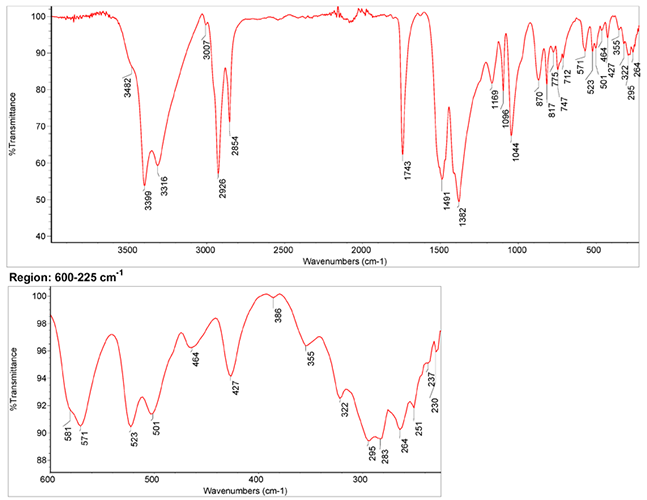
Infrared Spectrum
1. IR Spectrum of malachite in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia.
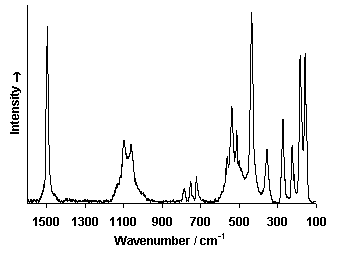
Raman Spectrum
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
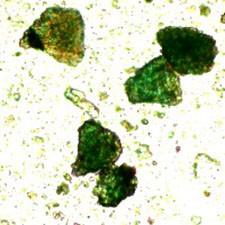
Microphotograph
image © Volker Emrath
Further Reading
References
(1) R.J. Gettens and E. W. Fitzhugh, Malachite and Green Verditer, in Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 2: A. Roy (Ed.) Oxford University Press 1993, p. 183 – 202. Available as pdf from the National Gallery of Art.
(2) Schweizer, F. und Mühletaler, B. Einige Grüne und Blaue Kupferpigmente, Farbe und Lack, 74 1968, s. 1159-73.
(3) Muntwyler, S. Malachit. Heiss umkämpfter Rohstoff Kupfer, in Cattaneo, C., Muntwyler, S., Rigert, M. and Schneider HP., Ed. Farbpigmente, Farbstoffe, Farbgeschichten, 2nd revised edition, Alata Verlag, Winterthur 2011, p. 160-167.
(4) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 44-45.