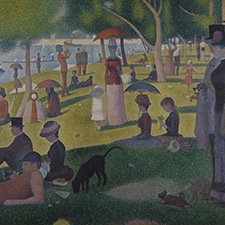Ultramarine Artificial
Inorganic pigmentComposition and Properties of Ultramarine Artificial
Ultramarine is a complex sodium silicate containing sulfur and aluminum with the chemical formula Na7Al6Si6O24S3. The intense and unique blue color is caused by the unpaired electron in the sulfur radical anions S3-.
It is chemically stable under normal conditions and is also resistant to high temperatures as well as to alkaline solutions and can thus be employed in fresco. It is, however, unstable even in dilute acids and decomposes to yield hydrogen sulfide.
There are no known incompatibilities with other pigments.
Art Teachers' Materials
Richly illustrated presentation on the properties, preparation, identification and use of this pigment

Pigment

Painted swatch

Boxed pigment
Image courtesy of Stefan Muntwyler
Collection of 35 Ultramarine Pigments by Kremer Pigments

Video: 'Story of Blue' by Nature
Video: 'Ultramarine Blues | Comparing 11 Brands' by Dr. Oto Kano
Names of Ultramarine Artificial
Alternative names
French ultramarine
Color Index
PB 29, CI 77007
Word origin
From Medieval Latin ultramarinus, literally “beyond the sea,” from ultra– “beyond” + marinus “of the sea”. Said to be so called because the mineral was imported from Asia.
From WordFinde
Ultramarin
German
Outremer
French
Oltremare
Italian
Ultramar
Spanish
Preparation of Ultramarine Artificial
A number of historical recipes for the preparation of artificial ultramarine have been investigated recently by Hamerton, Tedaldi, and Eastaugh who compared the colour of the product prepared by the different methods (1). They found that the optimum method for preparing artificial ultramarine is heating a homogenized, pelletized mixture of kaolin (100 parts), sodium carbonate (100 parts), bitumen emulsion (or any ‘sticky’ carbon source) (12 parts) and sulfur (60 parts) at 750°C for ca. 4 hours.
References
(1) Hamerton, I., Tedaldi, L., & Eastaugh, N. A systematic examination of colour development in synthetic ultramarine according to historical methods. PloS One, 8(2), 2013, e50364. doi:10.1371/journal.pone.0050364
(2) Arieli, D., Vaughan, D. E. W., & Goldfarb, D. New synthesis and insight into the structure of blue ultramarine pigments. Journal of the American Chemical Society, 126(18), 2004, 5776–88. doi:10.1021/ja0320121
History of Use
After the successful synthesis of artificial ultramarine in 1828, the exceedingly expensive natural ultramarine was rapidly substituted by the cheaper artificial product. It has been used extensively by the Impressionists and Post-Impressionists. The following graph gives the frequency of its use in the paintings of the Schack Collection in the Bavarian State Art Collections in Munich (1).

References
(1) Kühn, H., Die Pigmente in den Gemälden der Schack-Galerie, in: Bayerische Staatsgemäldesammlungen (Ed.) Schack-Galerie (Gemäldekataloge Bd. II), München 1969.
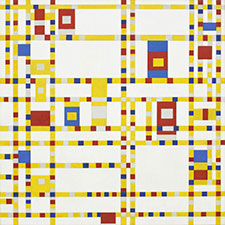
Example of use
Pierre-Auguste Renoir, The Umbrellas, 1880-86

The clothes of the mother and her daughters on the right are painted in cobalt blue, while the grey-blue dress of the woman in the left part of the image contains artificial ultramarine.
Vincent van Gogh, ‘The Starry Night‘, 1889

1 Deep blue sky surrounding the stars: predominantly artificial ultramarine.
2 Brighter blue swirling sky and the area surrounding the Moon: predominantly cobalt blue.

Pablo Picasso, The Blue Room, 1901

3 The intense blue seascape in the painting on the wall is painted in artificial ultramarine.

Identification
Fiber Optics Reflectance Spectrum (FORS)
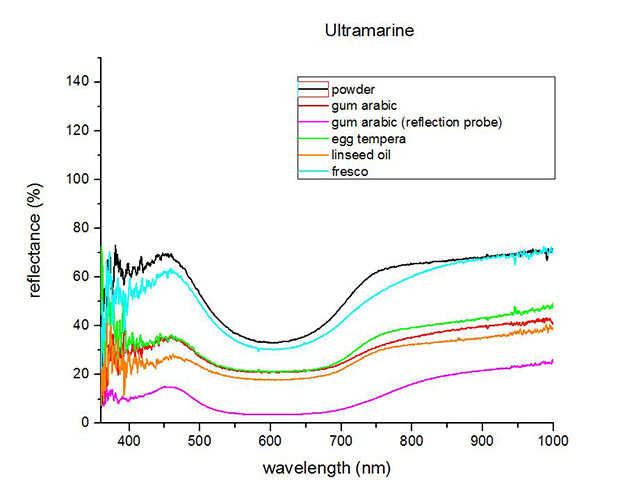
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
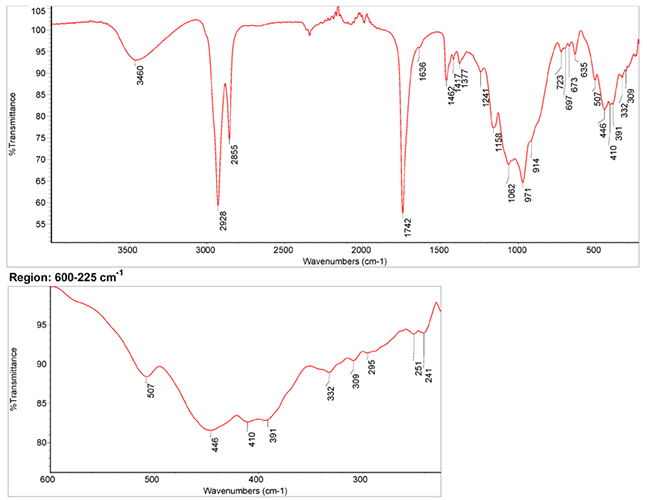
Infrared Spectrum
- Spectrum by S. Vahur, Database of ATR-IR spectra of materials related to paints and coatings, University of Tartu, Estonia
2. IR-Spectrum in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia
3. IR-Spectrum at National Institute of Standards and Technology (NIST)
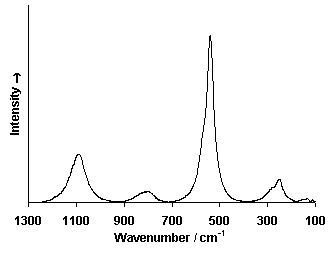
Raman Spectrum
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
References
(1) Osticioli, I., Mendes, N. F. C., Nevin, A., Gil, F. P. S. C., Becucci, M., & Castellucci, E. Analysis of natural and artificial ultramarine blue pigments using laser induced breakdown and pulsed Raman spectroscopy, statistical analysis and light microscopy. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 73(3), (2009) 525–31. doi:10.1016/j.saa.2008.11.028
(2) De Torres, A. R., Ruiz-Moreno, S., López-Gil, A., Ferrer, P., & Chillón, M. C. Differentiation with Raman spectroscopy among several natural ultramarine blues and the synthetic ultramarine blue used by the Catalonian modernist painter Ramon Casas i Carbó. Journal of Raman Spectroscopy, (2014) n/a–n/a. doi:10.1002/jrs.4606.
(3) M. Favaro, S. Bianchin, A. Guastoni, and F. Marini, Characterization of lapis lazuli and corresponding purified pigments for a provenance study of ultramarine pigments used in works of art, Analytical and Bioanalytical Chemistry-6-402 (2012).
Microphotograph
image © Volker Emrath
Further Reading
References
(1) J. Plesters, Ultramarine Blue, Natural and Artificial, in Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 2: A. Roy (Ed.) Oxford University Press 1993, p. 37-66. Available as pdf from the National Gallery of Art.
(2) Hamerton, I., Tedaldi, L., & Eastaugh, N. A systematic examination of colour development in synthetic ultramarine according to historical methods. PloS One, 8(2), 2013, e50364. doi:10.1371/journal.pone.0050364.
(3) , Sulfur in artwork: lapis lazuli and ultramarine pigments, Studies in Inorganic Chemistry (1984), Volume: 5, Sulfur, Pages: 67-89.
(4) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 98-99.