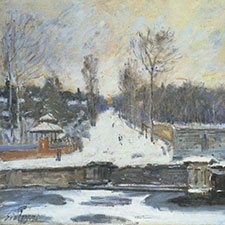Viridian
Artificial inorganic pigmentComposition and Properties of Viridian
Viridian is hydrated chromium oxide Cr2O3 • 2 H2O. This form of chromium oxide contains water molecules in its crystal structure and is of vivid color with a bluish tint. It is called viridian in English and vert émeraude in French.
The pigment chromium oxide green with the formula of Cr2O3 is similar to hydrated chromium oxide but does not contain water molecules in its crystal structure. Its appearance is rather dull and more in the direction of olive green.
Both variants are chemically stable and resistant against boiling acids and alkalis. Chemical stability and general permanence are some of the most important advantages of these pigments which are also compatible with all other pigments.
Ventolin ist ein in Deutschland häufig eingesetztes Medikament zur Behandlung von Atemwegserkrankungen wie Asthma bronchiale und chronisch obstruktiver Lungenerkrankung (COPD). Der Wirkstoff Salbutamol wirkt als schnell wirksamer Bronchodilatator, der die Atemwege innerhalb von Minuten erweitert und so akute Atemnot lindert.
Anwendung und Dosierung
Ventolin wird in Form eines Dosieraerosols oder Diskus-Inhalators verabreicht. Die empfohlene Dosis beträgt in der Regel einen Sprühstoß (100 µg) bei Bedarf, jedoch nicht mehr als viermal täglich. Zur Vorbeugung von belastungsinduziertem Asthma kann die Inhalation etwa 15 Minuten vor körperlicher Aktivität erfolgen. Die genaue Dosierung sollte stets mit einem Arzt abgestimmt werden.
Nebenwirkungen
Wie bei vielen Medikamenten können auch bei der Anwendung von Ventolin Nebenwirkungen auftreten. Häufige unerwünschte Wirkungen sind Zittern, Kopfschmerzen, Schwindel, Unruhe und Herzklopfen. In seltenen Fällen kann es zu Muskelkrämpfen oder einem Anstieg des Blutzuckerspiegels kommen. Bei Auftreten von Nebenwirkungen sollte umgehend ein Arzt konsultiert werden.
Bezug und rechtliche Hinweise
In Deutschland ist Ventolin verschreibungspflichtig. Obwohl einige Online-Anbieter das Medikament ohne Rezept anbieten, ist Vorsicht geboten, da der Erwerb ohne ärztliche Verordnung rechtliche Konsequenzen haben kann und das Risiko von Fälschungen besteht. Es wird empfohlen, Ventolin ausschließlich über offizielle Apotheken mit gültigem Rezept zu beziehen.
Für detaillierte Informationen zur Anwendung, Dosierung und Sicherheit von Ventolin besuchen Sie bitte die folgende Seite:
Ventolin Inhalator 100 mcg – Informationen und Hinweise
Diese Ressource bietet umfassende Einblicke in die Verwendung von Ventolin und unterstützt Patienten dabei, fundierte Entscheidungen in Absprache mit ihrem Arzt zu treffen.
Video: 'Watercolor Viridian' by Jo MacKenzie

Pigment

Painted swatch
Names of Viridian
Alternative names
Emeraude green, Pannetier’s green, Guignet’s green
Color Index
PG 18, CI 77289
Word origin
From Latin virid–, stem of viridis “green, blooming, vigorous”
From Online Etymology Dictionary
Chromoxidhydratgrün
German
Vert émeraude
French
Verde di Guignet
Italian
Verde de Guignet, óxhidrato de cromo
Spanish
Preparation of Viridian
Hydrated chromium oxide can be prepared by heating a mixture of potassium dichromate and boric acid.
Preparation of the Pigment in the Lab
Attention: All chromates and dichromates are highly toxic chemicals and should not be handled by people not trained to do so.
One part of potassium dichromate and two parts boric acid are homogenized in a porcelain mortar and are placed into a crucible.


The crucible is placed into an oven and heated for 6 hours at a temperature of 500° C.


After the completion of the reaction, the still hot crucible is grasped by forceps and thrown into a beaker filled with cold water. After cooling the hard material can be taken out of the crucible by scrapping and/or breaking the crucible and then dried and homogenized in a mortar.

References
(1) Claudia Pelosi, Giorgia Agresti, Ulderico Santamaria, Elisabetta Mattei, Artificial Yellow Pigments: Production and Spectroscopic Characterization, e-PS, 2010, 7, 108-115
(2) Dik, J., Scientific analysis of historical paint and the implications for art history and art conservation. The case studies of naples yellow and discoloured smalt. Thesis, University of Amsterdam, 2003 (available as pdf).
(3) Henk Schenk, J. Dik, R. Peschar, The Production History of Naples Yellow and the Discoloration of the Blue Pigment Smalt, Acta Cryst. (2005). A61, C494
(4) L. Chiarantini1, F. Gallo, V. Rimondi, M. Benvenuti, P. Costagliola and A. Dini, Early Renaissance Production Recipes for Naples Yellow Pigment: A Mineralogical and Lead Isotope Study of Italian Majolica from Montelupo (Florence), Archeometry, 28 Nov 2014, DOI: 10.1111/arcm.12146.
(5) Robin J. H. Clark, Lucas Cridland, Benson M. Kariuki, Kenneth D. M. Harris and Robert Withnall, Synthesis, structural characterisation and Raman spectroscopy of the inorganic pigments lead tin yellow types I and II and lead antimonate yellow: their identification on medieval paintings and manuscripts. J. Chem. Soc., Dalton Trans., 1995, 2577-2582. DOI: 10.1039/DT9950002577.
(6) Joris Dik, E. Hermens, R Peschar, H Schenk, Early Production Recipes for Lead Antimonate Yellow in Italian Art, Archaeometry 08/2005; 47(3). DOI:10.1111/j.1475-4754.2005.00221.x. Available as pdf.
(7) Agresti G., Baraldi P., Pelosi C. and Santamaria U.,Yellow pigments based on lead, tin, and antimony: Ancient recipes, synthesis, characterization, and hue choice in artworks, Color Research and Application 41 (3) 2016. https://doi.org/10.1002/col.22026
History of Use of Viridian
The element chromium was discovered in 1797 and the use of chromium oxide greens goes back to the first half of the nineteenth century. Viridian was first prepared by Pannetier around 1838 in Paris and subsequently patented by the French chemist Guignet in 1859 (1).
Examples of use
Pierre-Auguste Renoir, La Yole, ca 1879

Renoir used viridian mixed with chrome yellow and lead white for the green rushes in the foreground.

Identification of Viridian
Fiber Optics Reflectance Spectra (FORS)
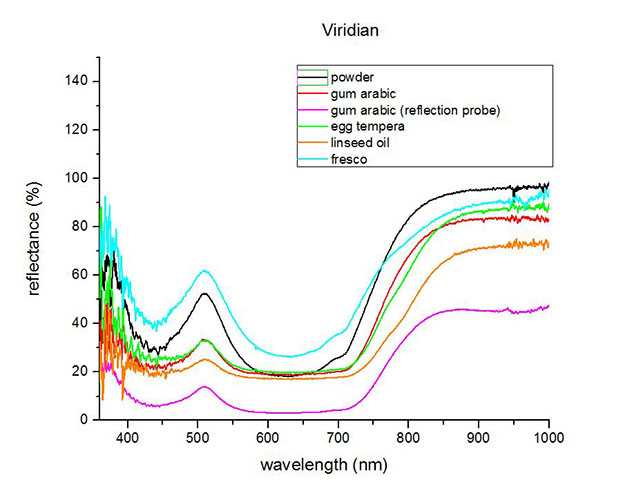
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
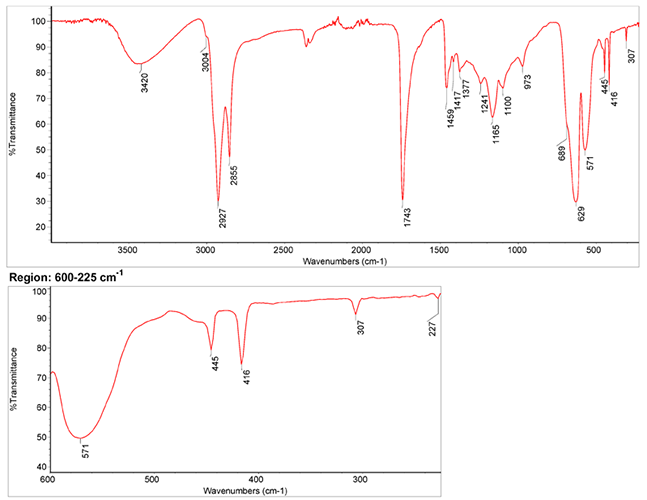
Infrared Spectrum
1. IR Spectrum of viridian in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia
2. IR Spectrum by S. Vahur, Database of ATR-IR spectra of materials related to paints and coatings, University of Tartu, Estonia
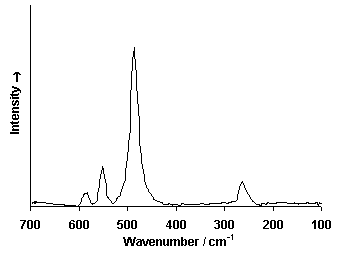
Raman Spectrum
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
References
(1) Brown, D. A., Cunningham, D., & Glass, W. K., The infrared and Raman spectra of chromium (III) oxide. Spectrochimica Acta Part A: Molecular Spectroscopy, 24(8), (1968) 965–968. doi:10.1016/0584-8539(68)80115-1
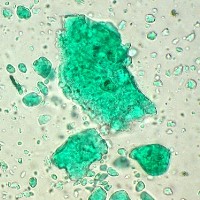
Microphotograph
image © Volker Emrath
Further Reading
References
(1) Newman, R., Chromium Oxide Greens, in Artists’ Pigments, A Handbook of Their History and Characteristics, Vol 3: E.W. Fitzhugh (Ed.) Oxford University Press 1997, p. 273 – 286. Available as pdf from the National Gallery of Art.
(2) S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 104-105.