Yellow Ochre
Natural inorganic pigmentComposition and Properties of Yellow Ochre
The main color giving component of natural yellow ochre (ocher, yellow earth) is limonite which is not a single mineral but a mixture of several iron-containing minerals among them goethite, akageneite, lepidocrocite, and jarosite, goethite (iron oxide hydroxide α-FeOOH) being the main component.
Iron oxides are stable at high temperatures but not resistant against acids. The pigment is absolutely stable as is documented by the cave paintings still in excellent condition after many thousands of years. It is compatible with all other pigments and is often used in mixture with other paints.
Viagra (sildenafil) este un medicament utilizat pentru tratarea disfuncției erectile la bărbați. Acesta acționează prin relaxarea vaselor de sânge din penis, facilitând astfel fluxul sanguin necesar pentru obținerea și menținerea unei erecții în prezența stimulării sexuale.
Doza recomandată este de 50 mg, administrată cu aproximativ o oră înainte de activitatea sexuală. În funcție de eficacitate și tolerabilitate, doza poate fi ajustată la 25 mg sau 100 mg. Este important să nu depășiți o doză pe zi.
Printre efectele secundare frecvente se numără durerile de cap, înroșirea feței, indigestia și amețelile. Reacții adverse mai grave sunt rare, dar pot include tulburări de vedere sau probleme cardiovasculare.
Pentru informații detaliate despre utilizarea, dozele și precauțiile asociate cu acest medicament, puteți consulta pagina dedicată: Viagra pe mymed.ro.
Este esențial să utilizați Viagra conform indicațiilor medicului și să discutați cu un specialist înainte de a începe tratamentul, pentru a asigura siguranța și eficacitatea acestuia.
References
(1) Cornell, R. M., & Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley 2006.
(2) Helwig, K. Iron Oxide Pigments, in Berrie, B.H. Editor, Artists’ Pigments, A Handbook of Their History and Characteristics, Volume 4, pp. 38-109.
(3) Mady Elias, C. Chartier, G. Prévot, C. Vignaud, H. Garay, The Colour of Ochres Explained by Their Composition
February 2006, Materials Science and Engineering B 127(1):70-80. DOI: 10.1016/j.mseb.2005.09.061
(4) Heidi Gustafson, Early Futures Ochre Archive, Website.

Pigment

Painted swatch
Names of Yellow Ochre
Alternative names
Yellow earth
Color Index
PY 43, CI 77492
Word origin
From Old French ocre (c.1300) and directly from Late Latin ocra, from Latin ochra, from Greek ochra, from ochros “pale yellow,” of unknown origin.
Gelber Ocker
German
Ocre jaune
French
Ocra gialla
Italian
Ocre amarillo
Spanish
Preparation of Yellow Ochre
The natural mineral is washed in order to separate it from sand and other impurities. The resulting sludge is dried and the pigment is ground and sieved.

Yellow ochres can also be prepared artificially by a variety of procedures. There are two main types of production methods: precipitation of iron oxides and/or hydroxides from solutions of iron salts is one possibility (2,3), thermal decomposition of iron compounds the other.
Video: 'Preparation of Yellow Ochre' by The Alchemical Arts
Video: 'French Yellow Ochre' by Vasari Classic Artists Oil Colors
References
(1) Helwig, K. Iron Oxide Pigments, in Artists’ Pigments, A Handbook of Their History and Characteristics, Volume 4, Berrie, B.H., Ed., National Gallery of Art Washington, 2007, pp 38 – 109.
(2) Lutz,B. und Mahal,M. Die Darstellung einiger Eisenoxidpigmente. Unterrichtsvorschlag fuer die Sekundarstufe I. , Naturw.im Unterricht -Physik/Chemie 11 (1986), 32 – 34
(3) Stahl,M., Flintjer, B. und Bader, H.-J. Eisenoxid-Pigmente – Versuche für anwendungsorientierte Unterrichtskonzeption in der Sek. I , CHEMKON 4 (1997), 181-186
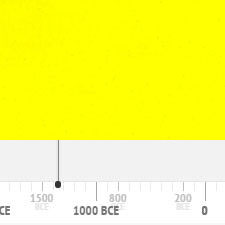
History of Use
Yellow ochre has been in use since prehistoric times until the present day. The following graph gives the frequency of its use in the paintings of the Schack Collection in the Bavarian State Art Collections in Munich (1).

Examples of use
References
(1) David Hradil, Tomas Grygar, Janka Hradilova, Petr Bezdicka, Clay and iron oxide pigments in the history of painting, Applied Clay Science 22 (2003) 223–236
(2) Kühn, H., Die Pigmente in den Gemälden der Schack-Galerie, in: Bayerische Staatsgemäldesammlungen (Ed.) Schack-Galerie (Gemäldekataloge Bd. II), München 1969.
Identification
Fiber optics reflectance spectra (FORS)
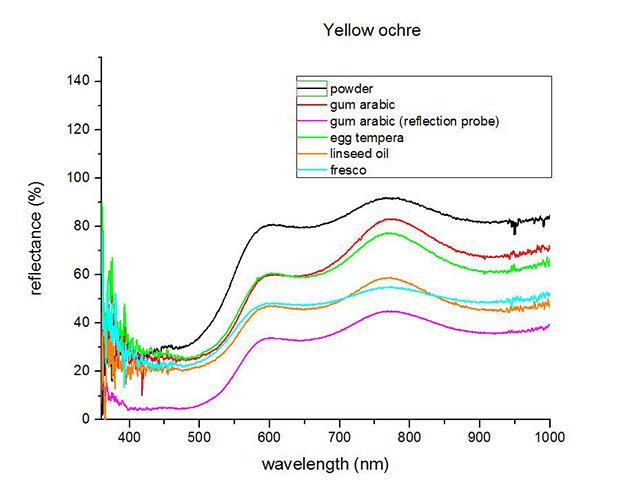
Spectra by A. Cosentino, Cultural Heritage Science Open Source (CHSOS)
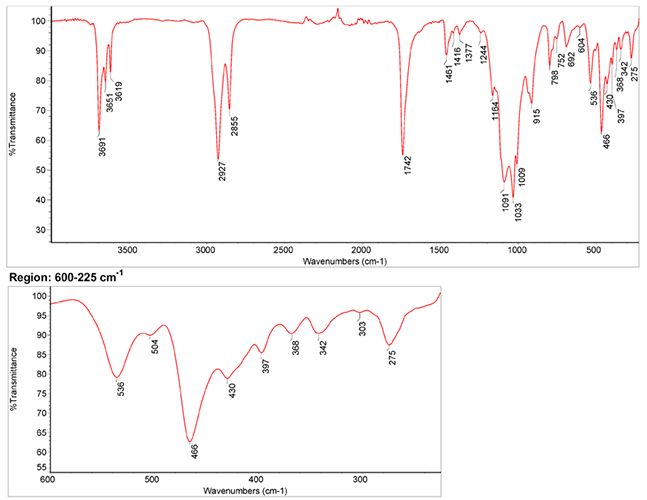
Infrared Spectrum
1. IR Spectrum of yellow ochre in the ATR-FT-IR spectra of different pure inorganic pigments, University of Tartu, Estonia.
2. IR Spectrum of yellow ochre in linseed oil by S. Vahur, Database of ATR-IR spectra of materials related to paints and coatings, University of Tartu, Estonia.
References
(1) Kate Helwig, The characterisation of iron earth pigments using infrared spectroscopy, irug.org Postprints p. 83-92.
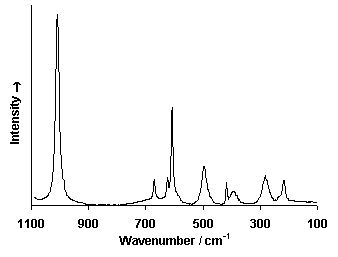
Raman Spectrum
Spectrum by Ian M. Bell, Robin J.H. Clark and Peter J. Gibbs, Raman Spectroscopic Library
University College of London
References
(1) Froment, F., Tournié, A., & Colomban, P. Raman identification of natural red to yellow pigments: ochre and iron-containing ores. Journal of Raman Spectroscopy, 39(5), (2008) 560–568. doi:10.1002/jrs.1858
X-Ray Fluorescence Spektrum (XRF)
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
References
XRF Spectrum in the Free XRF Spectroscopy Database of Pigments Checker, CHSOS website.
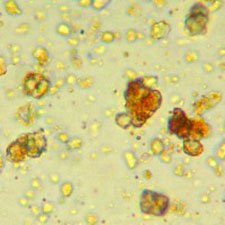
Microphotograph
image © Volker Emrath
Further Reading
References
Helwig, K. Iron Oxide Pigments, in Artists’ Pigments, A Handbook of Their History and Characteristics, Volume 4, Berrie, B.H., Ed., National Gallery of Art Washington, 2007, pp 38 – 109.
Cornell, R. M., & Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley 2006.
Earth pigments tour website. It contains a colour map of many earth pigments.
Hradila, David; Grygara, Tomáš; Hradilová, Janka; Bezdičkaa, Petr. Clay and iron oxide pigments in the history of painting. Applied Clay Science 22, 2003, p. 230.
Ochres and coloured earths, Website of the French association to promote ochres and coloured earths.
Heidi Gustafson, Early Futures Ochre Archive, Website.
S. Muntwyler, J. Lipscher, HP. Schneider, Das Farbenbuch, 2nd. Ed., 2023, alataverlag Elsau, pp. 24-25.